Haloalkanes are the organic compounds containing the halogen group attached to the carbon. The halogen bonded to the carbon atom acts as a functional group of haloalkane. eg:
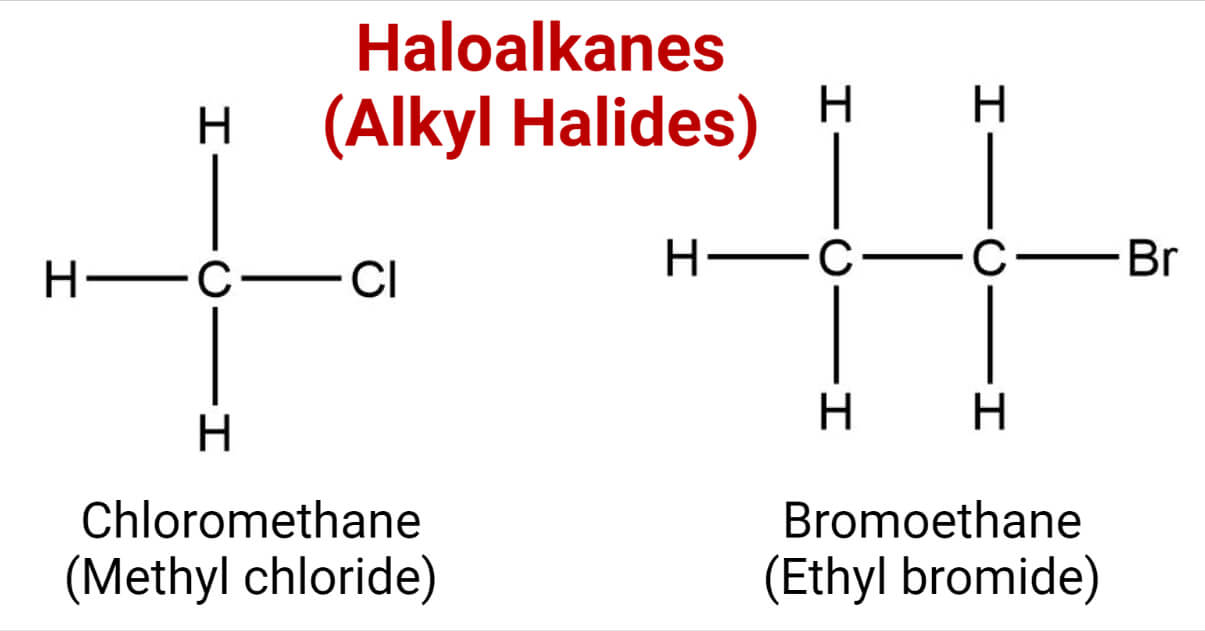
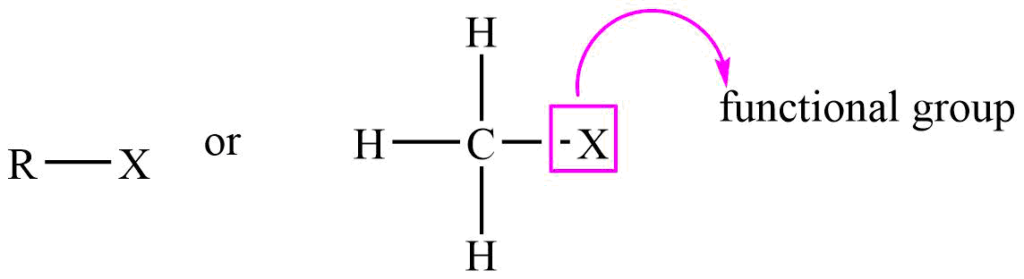
They have the general formula R-X. where R= alkyl group, X= Cl, Br, I, and F.
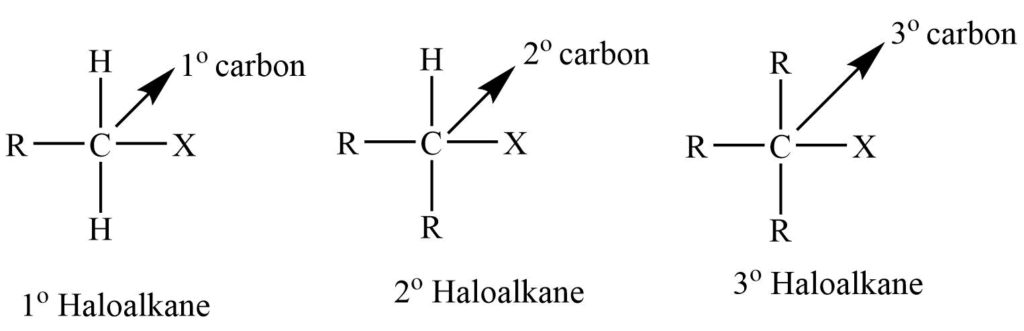
Depending upon whether the X atom is attached to the primary (1o), secondary (2o), and tertiary (3o), haloalkanes are classified as primary, secondary, and tertiary haloalkanes.
Interesting Science Videos
Haloalkanes Structure
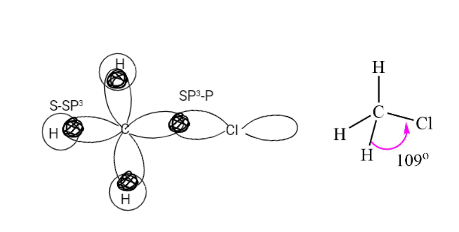
For eg: Chloromethane, in Chloromethane, the C-Cl bond is formed by the overlap of the SP3 orbital of carbon and the half-filled P orbital of chlorine. Each C-H bond is formed by overlapping of SP3 orbital of carbon and the S orbital of hydrogen.
Haloalkanes Nomenclature
There are two methods for the naming of haloalkanes.
1. Common name system: In this system, the alkyl group attached to halogen is named first, which is followed by the appropriate name of halogen group like bromide, chloride, iodides
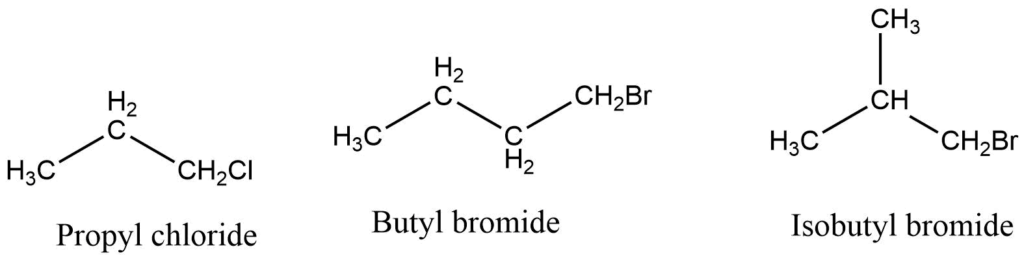
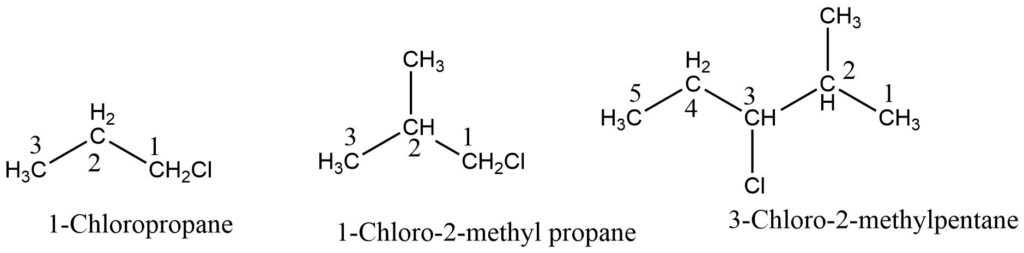
2. IUPAC system:
- Select the longest chain carbon-containing the halogen group
- Number the chain so that the carbon-containing halogen atom gets the lowest number.
- Name the halogen group and indicate the position of halogen by numbers.
- Indicate the position of other substituents by the numbers and assign an appropriate name to them.
- Prefix their name to the name of the parent chain
General methods of preparation of Haloalkanes
1. From alcohol
Reaction of alcohol with halogen acids
Halogen acids like HBr, and HI react with alcohols to give bromoalkene and iodoalkane. While HCl reacts with alcohol in presence of ZnCl2 to give chloroalkane.
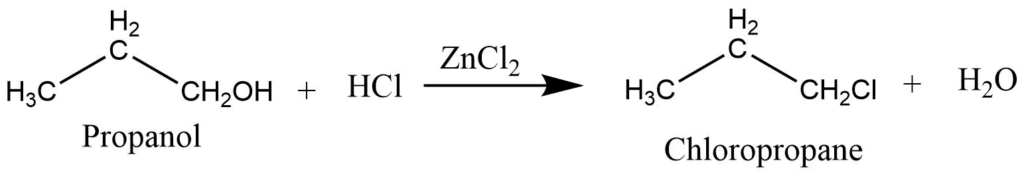
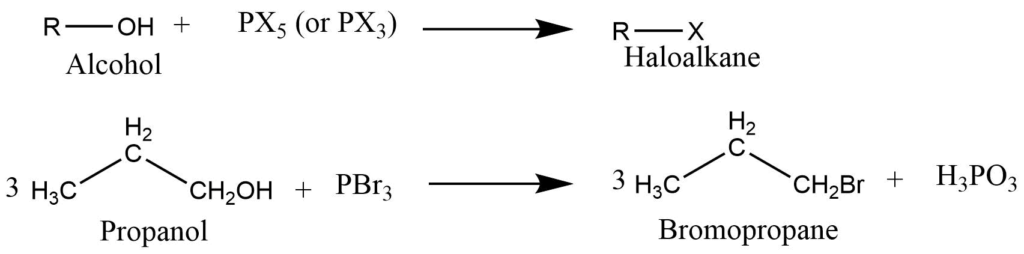
Reaction of alcohol with phosphorous halide
Alcohol on reaction with a phosphorous halide (PX5 or PX3) produces the haloalkanes.
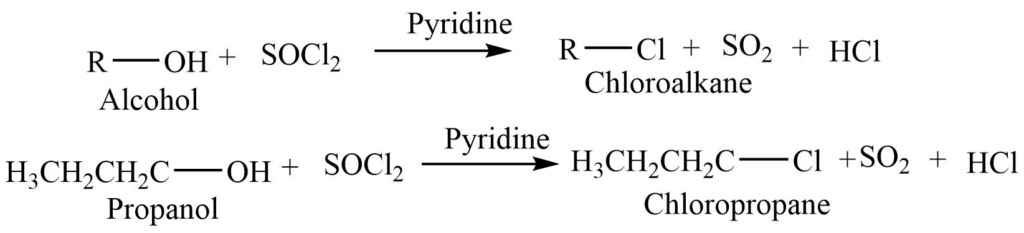
Reaction of alcohol with thionyl chloride
Alcohol reacts with thionyl chloride i.e., SOCl2 to give chloroalkane. This reaction takes place through the SNi mechanism.
2. Halogenation reaction
Halogenation of alkane
At high temperature (400oC) alkane reacts with Cl2 or Br2 in the presence of UV light to give haloalkane. Polyhalogen derivatives are also obtained along with haloalkane from this reaction.
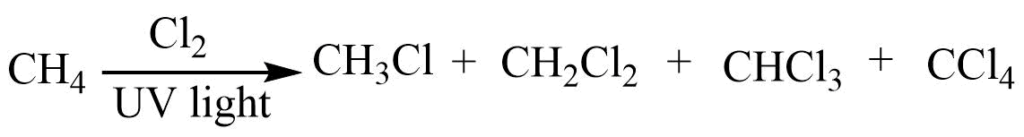
Halogenation of alkene
Halogen acids (HCl, HBr, HI) adds to the alkene to give haloalkane. This addition reaction follows the Markovnikov rule.

Exception: Addition of HBr to the alkene in presence of organic peroxide (R-O-O-R) gives an anti-Markovnikov product
(Anti-Markovnikov rule: when HBr reacts with an alkene in presence of organic peroxides, the proton is attached to the carbon atom with the lowest hydrogen atoms. It is also called peroxide effect or Kharasch effect)
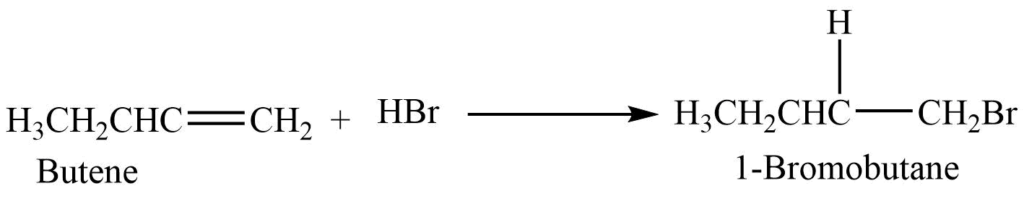
Halogen exchange reaction
Alkyl chloride or bromide heated with a concentrated solution of sodium iodide in presence of acetone gives alkyl iodide.

Alkyl fluoride can also be prepared by treating alkyl chloride or bromide with inorganic fluoride.

Laboratory Preparation of Haloalkane
Example- Bromoethane Preparation
In the laboratory, bromoethane can be synthesized by reacting ethyl alcohol with concentrated sulfuric acid in the presence of potassium bromide.
Reaction involved:
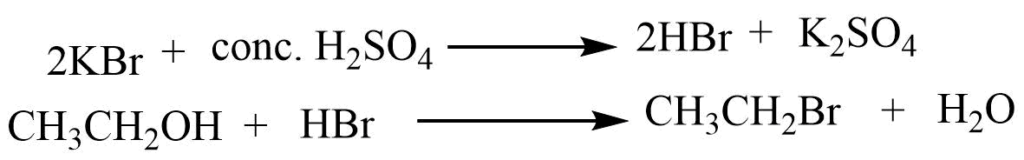

Physical Properties of Haloalkanes
- Alkyl halides are insoluble in water and soluble in an organic solvent. Their insolubility in water may be due to their inability to form a hydrogen bond.
- CH3Cl, CH3Br, CH3F, and CH3CH2Cl are gases at room temperature. The alkyl halides up to C18 are colorless liquids while alkyl halides beyond C18 are colorless solids.
- Due to the higher molecular weight haloalkanes have a higher boiling point than the alkanes of a comparable number of carbons. The boiling point of alkyl halide follows the order of RI> RBr> RCl> RF.
- In the infrared region, haloalkane shows strong absorption for stretching vibrations of the carbon-halogen bond. Characteristic absorption is observed at 950-1350 cm-1 for C-F, at 510-775 cm-1 for C-Cl, 490-650 cm-1 for C-Br and 465-600 cm-1 for C-I.
Chemical Properties of Haloalkanes
Haloalkanes are reactive compounds, that undergo the substitution, elimination, and reduction reaction. On reaction with metal haloalkanes from the organometallic compounds.
1. Substitution Reactions
SN1 reaction mechanism: It is unimolecular substitution reaction. It involves a two-step process
1st step: Alkyl halide ionizes to form a planar carbonium ion.
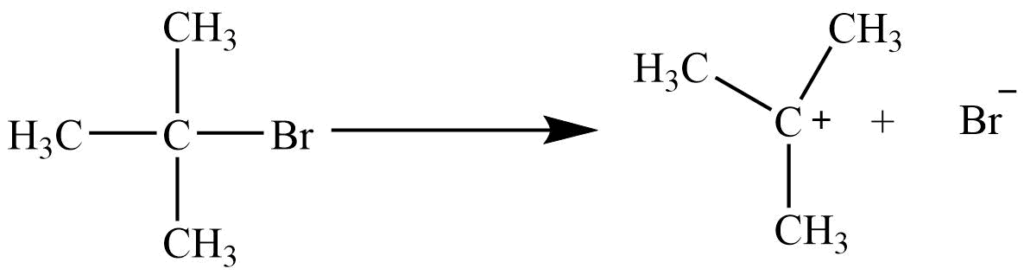
2nd step: Nucleophile attacks the planar carbonium ion to give a product.
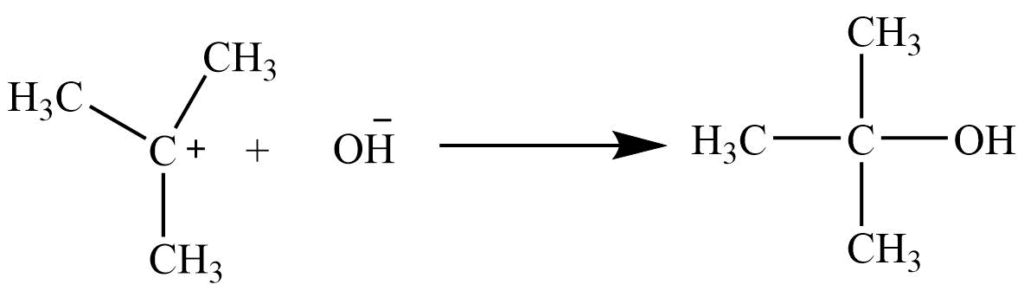
Generally, tertiary alkyl halide undergoes substitution reaction through the SN1 mechanism.
SN2 reaction mechanism: It is bimolecular substitution reaction. In this process, the attack of the nucleophile and the ejection of the halide group takes place at the same time. Generally, primary alkyl halide undergoes substitution reaction by the SN2 reaction mechanism.
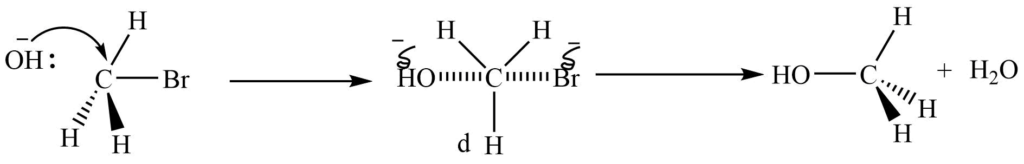
Some substitution reactions:
- Reaction with aqueous KOH: Haloalkane on reaction with aqueous KOH forms alcohol.
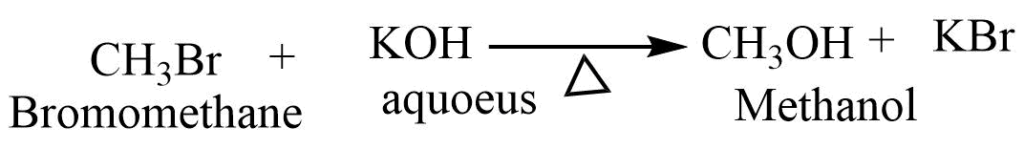
- Reaction with moist silver oxide: Moist silver oxide reacts with haloalkane to give alcohol.
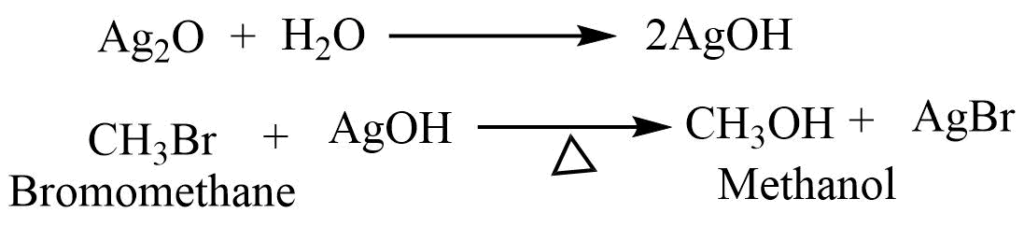
- Reaction with dry silver oxide: Haloalkane on heating the dry silver oxide forms the ether
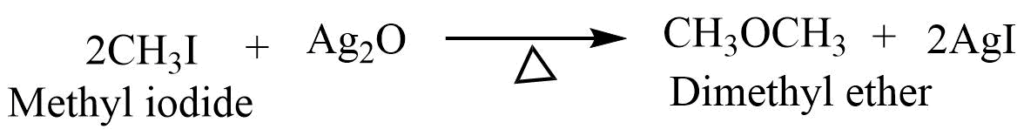
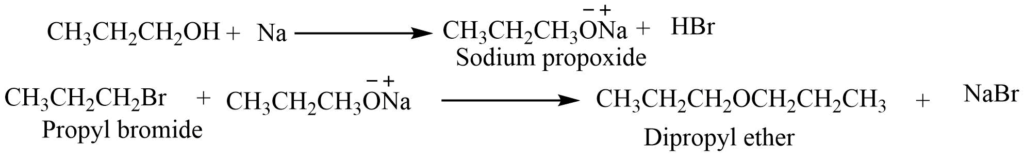
- Sodium alkoxide: Sodium alkoxide, formed by dissolving metallic sodium in excess of appropriate alcohol, reacts with haloalkane to form ether.
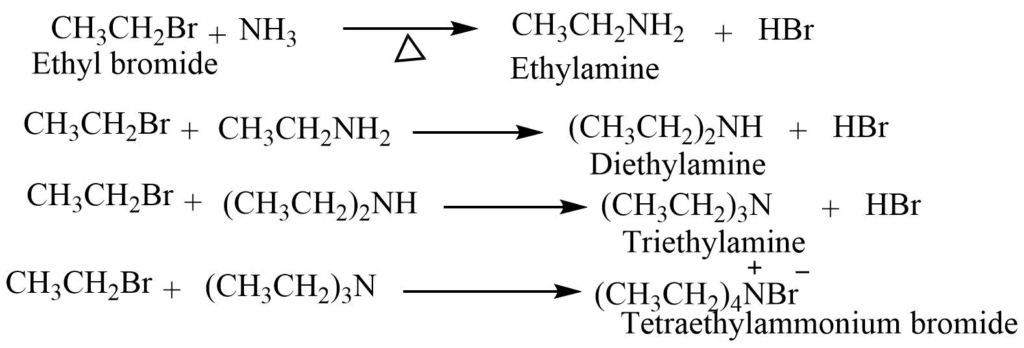
- Reaction With Ammonia: Alkyl halide when heated with an alcoholic solution of ammonia in a sealed tube gives amine.
2. Reduction reaction
In presence of catalysts like ZN/HCl, LiAlH4, and H2 in Ni or Pd haloalkanes reduce to alkane.
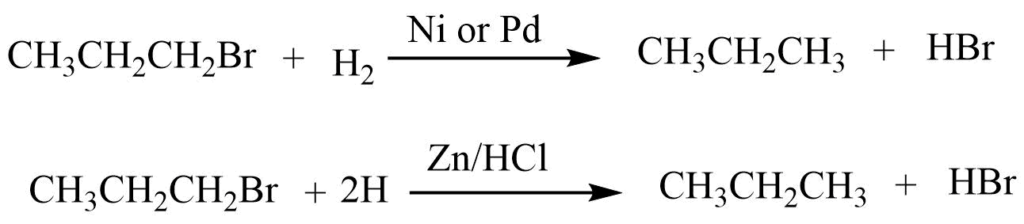
3. Elimination reaction
Haloalkanes react with the alcoholic KOH to form an alkene. It is also known as the dehydrohalogenation reaction.
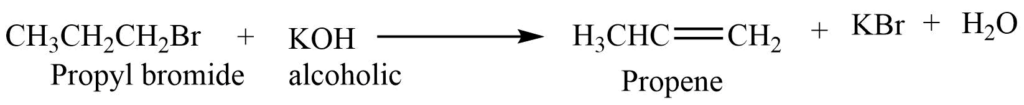
4. Some other reactions
- Reaction with Mg: Alkyl halide reacts with Mg metal in presence of dry ether to give Grignard reagents.

- Wurtz reaction: In the presence of dry ether, haloalkane reacts with sodium metal to produce an alkane with double the number of carbon atoms. Only symmetrical alkane can be synthesized from the wurtz reaction
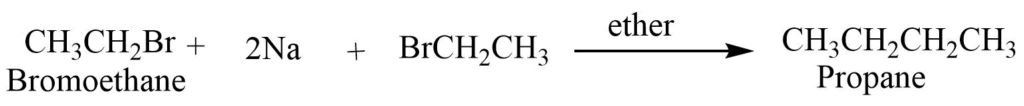
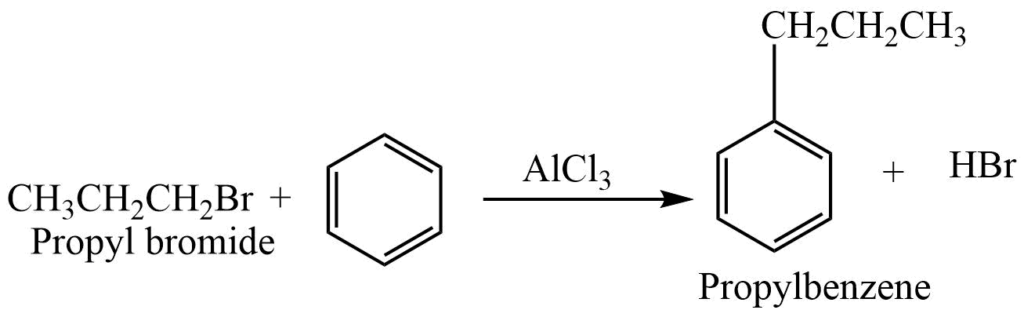
- Friedel Crafts Alkylation: In the presence of anhydrous AlCl3, haloalkane reacts with benzene to produce alkylbenzenes.
Uses of Haloalkanes
- They are used for organic synthesis reactions.
- Bromoethane is used as a solvent as well as an anesthetic.
- They can be used commercially as a fire extinguishers.
- They are used as degreasers and aerosol propellants.
- They are also used as refrigerants and fumigants.
References
- Arun Bahl, and B.S. Bahl (2006). A textbook of organic chemistry Chemistry. New delhi: S. CHAND.
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- https://byjus.com/chemistry/haloalkanes-haloarenes/
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alkyl_Halides/Properties_of_Alkyl_Halides/Haloalkanes
- https://sciencestruck.com/alkyl-halides-properties-uses
- https://www.embibe.com/exams/haloalkanes/
- https://byjus.com/chemistry/sn1-and-sn2-reaction-of-haloalkanes/
- .https://www.toppr.com/ask/content/story/amp/bromoethane-laboratory-and-commercial-preparation-81874/
