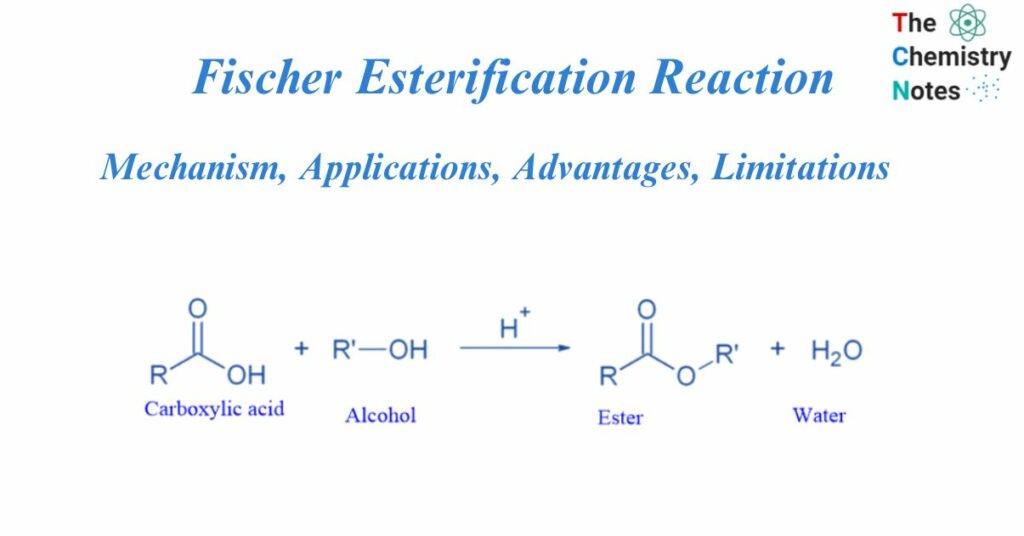
Fischer Esterification Reaction involves the reaction between an alcohol and carboxylic acid to form an ester. Emil Fischer and Arthur Speier initially explained the reaction process in 1895. Fischer esterification is a type of nucleophilic substitution process. It’s also known as Fischer-Speier Esterification.
This reaction frequently occurs in the presence of an excess of alcohol and has a slower rate of reaction. As a result, Fischer Esterification is a simply reversible reaction.
What is Fischer’s Esterification Reaction?
The acid-catalyzed condensation of a carboxylic acid with an alcohol to create esters is known as Fischer esterification. This is a reversible process that takes place in the presence of strong acids like sulphuric acid (H2SO4).
The presence of an acid catalyst in the ester synthesis pathway aids in two ways:
- It causes the carbonyl function (which makes the carbonyl carbon more electrophilic) to be nucleophilically attacked by the alcohol.
- The hydroxyl group is protonated to produce water, which is a better-leaving group.
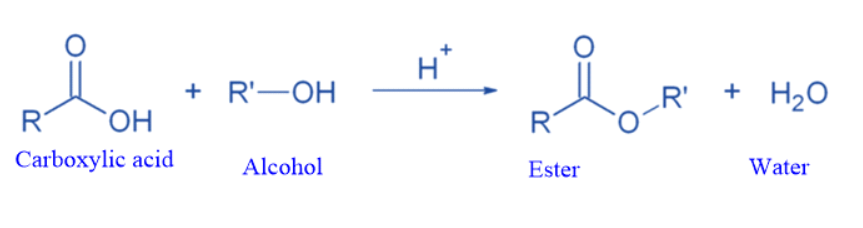
The esterification of carboxylic acids with alcohols by Lewis or Brnstedt acids is a typical reaction in which the products and reactants are in equilibrium.
The equilibrium can be changed by eliminating one product from the reaction mixture (for example, azeotropic distillation or absorption by molecular sieves) or by using an excess of one reactant.
Mechanism of Fischer Esterification Reaction
Step I: The acid catalyst protonates the carbon in the carboxylic acid. The addition of a hydrogen atom results in a positive charge on the oxygen atom. Protonation initiates the esterification process and opens the door for a nucleophilic attack.
Step II: The nucleophilic O-H group attacks the carbon atom of the carboxylic acid. This forms a connection between the O-R’ and carboxylic acid groups and neutralizes the charge of the positive oxygen atom. A positively charged oxonium ion has now been added to the O-R’ group. The R groups on this molecule can have a variety of structures.
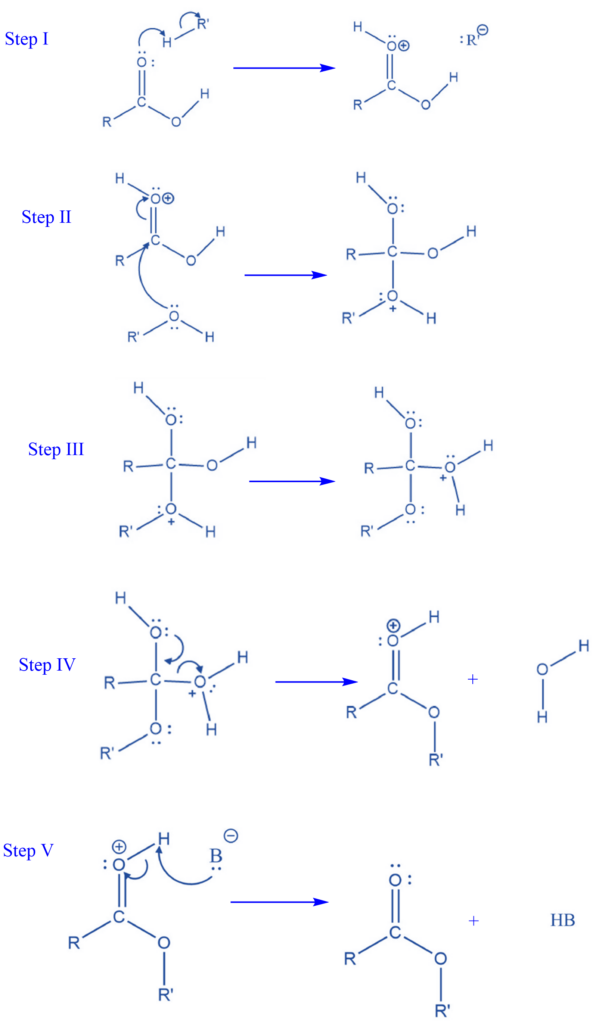
Step III: The intramolecular movement of hydrogen atoms is involved in this process. This is known as tautomerism.
Step IV: A water molecule is eliminated in this stage, resulting in a protonated ester.
Step V: The base then eliminates the proton, resulting in the creation of a neutral ester.
Factors Affecting Fischer Esterification Reaction
Several parameters can influence the Fischer Esterification process, including the concentration of the reactants, the reaction temperature, the type and amount of the acid catalyst, and the presence of any impurities. In general, increasing the concentrations of the reactants and the acid catalyst, as well as the reaction temperature, can speed up the reaction. Excessive heat, on the other hand, can cause the ester product to hydrolyze. Impurities in the reactants or the acid catalyst can also influence the reaction.
Applications of Fischer Esterification Reaction
- Esters are commonly used in synthetic flavoring, perfumes, and cosmetics. Esters are essential components of many produced items.
- If we have a molecule with both carboxylic and hydroxyl groups. A cyclic ester is generated during the intramolecular reaction. Lactones are another name for cyclic esters.
- Many flavors and scents are produced through esterification, such as isoamyl acetate, which offers banana flavor and aroma.
- Esterification of salicylic acid with acetic acid is a typical process for synthesizing medicines, including aspirin.
- Many plasticizers, such as DEHP, are produced via the Fischer Esterification Reaction and are used to improve the flexibility and durability of plastics.
- Ester, which has numerous synthetic and biological uses, is produced by Fischer Esterification Reaction. Esters, for example, are employed as solvents in lacquers, paints, and varnishes.
- It is useful for the preparation of several organic chemistry reagents, such as ethyl acetate, a typical solvent for reactions and extractions.
- The reaction technique produces fatty acid esters. They have a wide range of applications such as biodiesel fuel, food additives, and cosmetic products.
Advantages of Fischer Esterification Reaction
- In comparison to other esterification methods, it is rather simple.
- Unlike other esterification mechanisms, the chemicals employed and byproducts produced in this mechanism are non-toxic to the environment.
Limitation of Fischer Esterification Reaction
- This ester is generated in the presence of water, which must be continuously removed to prevent the reversible reaction. One of the disadvantages of Fischer esterification is the equilibrium between reactants and products.
- The reaction is acceptable for most carboxylic acids, but the alcohol should be a primary or secondary alkyl. Elimination is common with tertiary alcohols.
- Without a catalyst, the reaction becomes extremely slow.
- Fischer Esterification Reaction turns backward in the presence of a small quantity of water.
- The Fischer esterification process cannot produce phenol esters.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
