Esters are the derivatives of carboxylic acids containing -COOR as a functional group, in which the -OH group of a carboxylic acid is replaced by the -OR group.
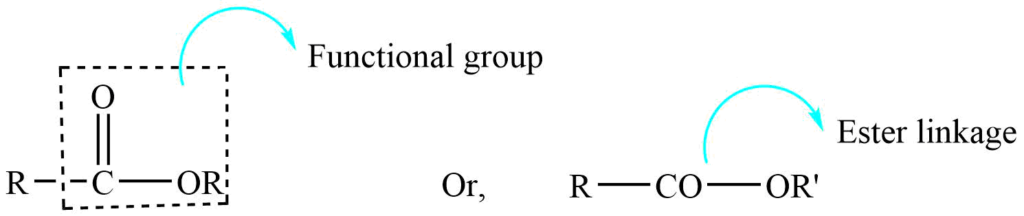
Esters are important derivatives of an acid. The ester of a carboxylic acid is commonly called a carboxylic ester. A large number of esters occur in flowers and fruit, which are responsible for their fragrance. They are used to make synthetic products like perfumes, pesticides, and solvents.
Nomenclature of Esters
The name of the alkyl group attached to the oxygen comes first in the common name system, followed by the name of the parent acid, with the suffix ‘-ic acid’ replaced by ate. While the suffix ‘ic acid’ is replaced by -oate in the IUPAC system.
| Formula | Common name | IUPAC name |
| RCOOR | Alkyl carboxylate | Alkyl alkanoate |
| HCOOCH3 | Methyl formate | Methyl methanoate |
| CH3CH2COOCH3 | Methyl propionate | Methyl propanoate |
Methods of preparation of Esters
1. By Fischer esterification: Reaction of a carboxylic acid with an alcohol in presence of an acid catalyst like H2SO4 or HCl produces ester.
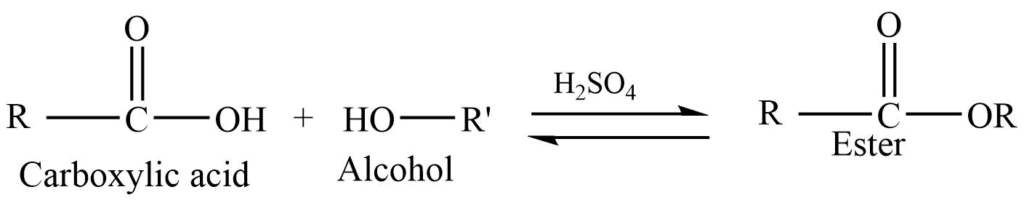
2. From acid chloride: Acid chloride reacts with alcohol in presence of pyridine to give ester.

3. From carboxylate salt: Sodium salt of carboxylic acid reacts with an alkyl halide to give ester.

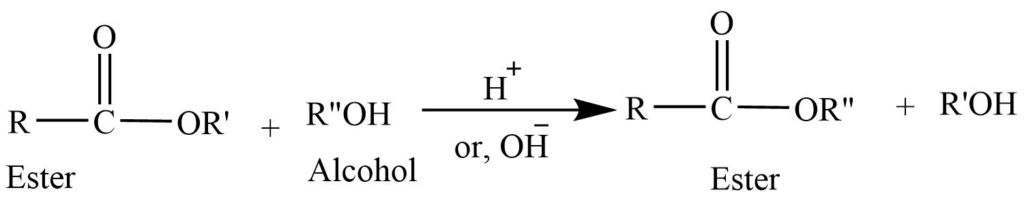
4. Transesterification reaction (Ester exchange): Ester of the alcohol reacts with another alcohol in presence of mineral acid to give the ester of the second alcohol.
5. By diazomethane: Carboxylic acid reacts with diazomethane to give ester. From this reaction, only methyl ester can be produced.

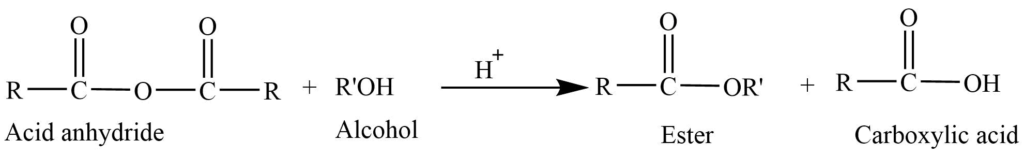
6. From acid anhydride: Acid anhydride reacts with alcohol to give ester.
Physical properties of Esters
- Lower carboxylic esters are colorless liquids with distinctive fruity odors.
- Esters are insoluble in water but soluble in organic solvents.
- The boiling point of methyl ethyl esters is significantly lower than that of parent acid.
- In the IR spectrum ester shows the characteristic -C=O stretching frequency at 1735 cm-1.
Chemical properties of Esters
The Carbonyl group of the ester is electron deficient carrying a positive charge on it Ester undergoes nucleophilic substitution reactions similar to acid halide and acid anhydride, but the reaction is slower due to the presence of the resonance effect.
Hydrolysis: Ester on heating with water in presence of H2SO4 undergo hydrolysis to give carboxylic acid and alcohol. This reaction is the reversible one.

- Reaction with ammonia: Reaction of the ester with ammonia in presence of solvent like ethanol gives amide.
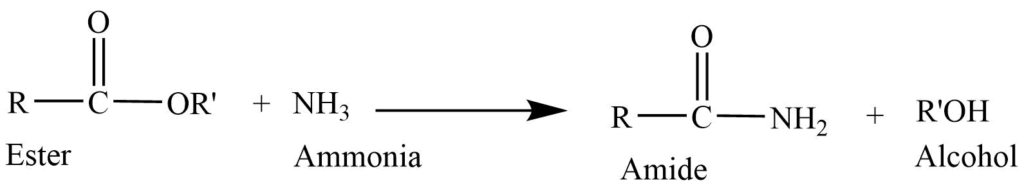
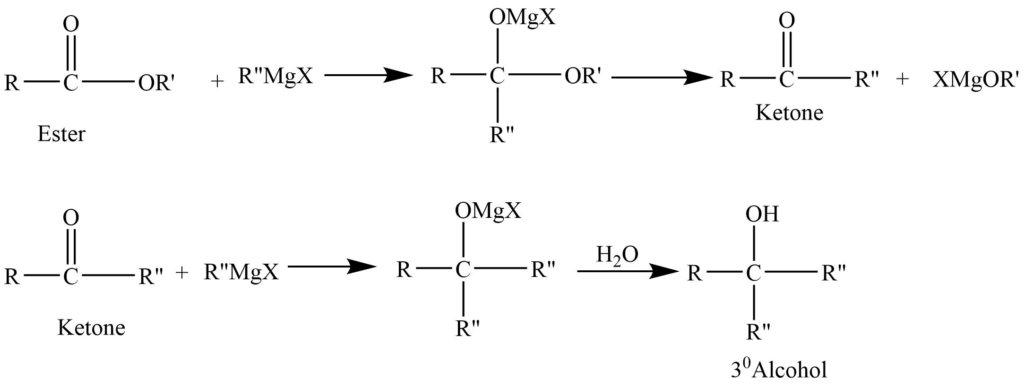
- Reaction with Grignard reagent: Ester reacts with one molecule of Grignard reagent to give ketone which on further reaction with another molecule of Grignard reagent produces tertiary alcohol.
- Condensation reaction (Claisen condensation reaction): In presence of a strong base (C2H5ONa), esters with α hydrogen undergo a condensation reaction to form β keto ester.
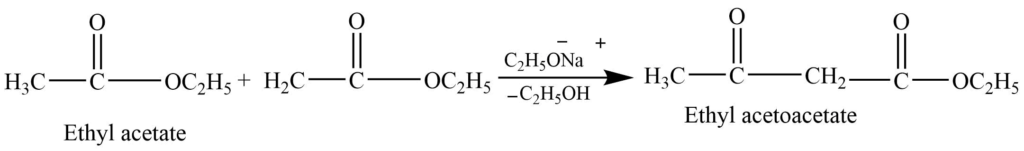
- Reduction to alcohols: On reaction with the reducing agents like LiAlH4 esters reduces to primary alcohols, corresponding to the acid from which it was derived.

Uses of Esters
- Esters are used in many organic synthesis reactions as solvents.
- Esters are used to prepare medicine for the treatment of diseases like asthma, and leprosy.
- They are used in soap, detergent, and cosmetics.
- Esters are used as the flavoring agent in foods and soft drinks.
- Phosphodiesters are the backbone of DNA molecules.
- Polyesters are used to make plastics.
- Esters are also used in synthetic lubricants.
References
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- https://www.pure-chemical.com/blog/esters-its-chemical-nature-properties-and-uses/
- https://www.britannica.com/science/ester-chemical-compound
- https://www.chemguide.co.uk/organicprops/esters/background.html
- https://byjus.com/chemistry/ester/
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_(Ball_et_al.)/15%3A_Organic_Acids_and_Bases_and_Some_of_Their_Derivatives/15.05%3A_Esters_-_Structures_and_Names
- https://sites.google.com/site/chemistryolp/uses-of-carboxylic-acid-and-esters
- https://www.scienceabc.com/pure-sciences/what-are-esters-formation-properties-and-uses.html

what is the use of the following substances in agriculture;glufosinate ammonium,pendimethalin,bentazone,clethodim