The dipole moment is a quantity that describes the separation of two opposite charges. It is a quantity that can be measured in the lab for a molecule and used to calculate the size of the partial charges on the molecule (assuming the bond length is known). It is defined as the product of the magnitude of the separated charge and the separation distance.
What is a dipole moment?
In chemistry, it expresses the polar nature or polarity of the molecules. It is defined as the product of charge and the distance between atoms in a chemical bond. It happens when there is a charge separation. A single atom or molecule has a dipole moment. When it occurs molecule, it might occur between two atoms in an ionic or covalent bond.
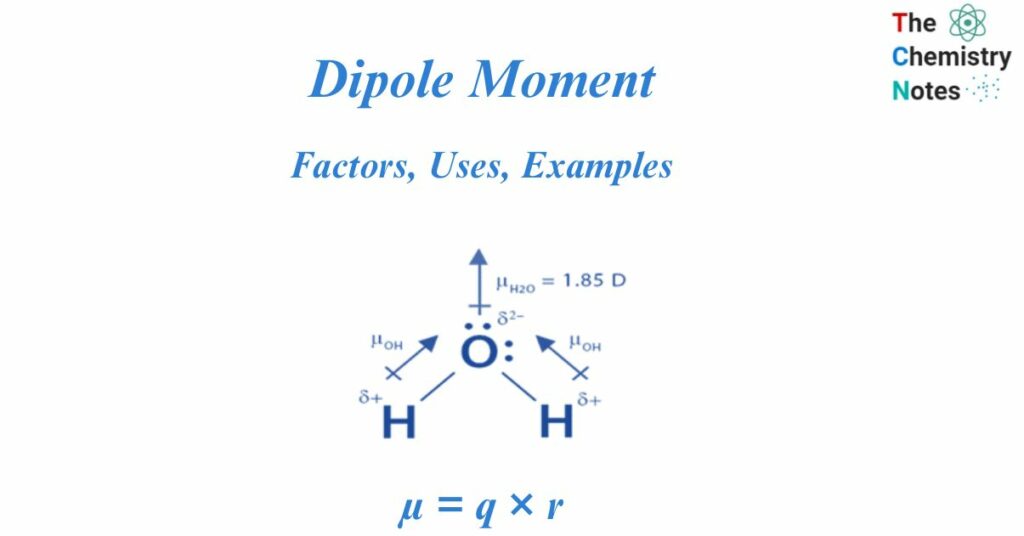
μ = q x r
Unit of dipole moment
The charge in the CGS system is given in esu and the bond length in cm. As a result, it is measured in esu cm. The charge is in the order of 10-10 esu, while the separation is in the order of 10-8 cm. As a result, the order is 10-18 esu cm. This magnitude is known as 1 Debye. As a result, 1 Debye = 10-18 esu cm.
The charge in the SI system is given in coulombs and the length in meters. As a result, the coulomb meter is it’s SI unit.
What are polar molecules?
Polarity occurs in molecules when the center of gravity of the positive charge does not coincide with the center of gravity of the negative charge. These are known as polar molecules. Polar molecules include hydrogen chloride, water, methyl chloride, and benzyl chloride.
Facts about the Dipole Moment
- The bond dipole moment is the dipole moment of a single bond in a polyatomic molecule, which differs from the molecule’s overall dipole moment.
- It is a vector quantity, which means it has both magnitude and definite directions. As it is a vector variable, it can be 0 because two opposing bond dipoles can cancel each other out.
- According to the convention, it is represented by a tiny arrow with its tail in the negative center and its head in the positive center.
- It is symbolized in chemistry by a modest variant of the arrow sign. A cross in the positive center and an arrowhead in the negative center represent the dipole moment. This arrow depicts the molecule’s shift in electron density.
- In the case of a polyatomic molecule, it is the vector sum of all bond dipoles.
Dipole moment in monoatomic species
In chemistry, it is used to specify the structure, bond angle, bond energy, and polarity of various compounds. Because the charge of the constituent atom is spread symmetrically, monoatomic noble gases like benzene are non-polar.
Dipole moment in homonuclear diatomic molecule
Because of their symmetrical charge distributions and equal electronegativity and ionization energy, homonuclear diatomic molecules such as nitrogen, oxygen, and chlorine have zero dipole values.
Dipole moment in heteronuclear species
A dipole/dipole moment does not exist in molecules with symmetrical/opposite dipoles. Because the bond polarities cancel each other out, there is no net dipole.
For example:
In the beryllium fluoride molecule, the bond angle between the two beryllium-fluorine bonds is 180o. As fluorine is a more electronegative atom than Beryllium, it causes the electron density to move towards itself. In a BeF2 molecule, the two individual bond dipole moments cancel each other out. The net dipole moment of a BeF2 molecule is 0 since they are equal in magnitude but opposite in direction.

Even though a molecule has symmetry, it still has a dipole moment. Because the oxygen atom is far more electronegative than the hydrogen atom, the electrons in a water molecule are concentrated around it. According to the Valence shell electron pair repulsion theory, the presence of a lone pair of electrons in the oxygen atom causes the water molecule to have a bent shape. As a result, unlike in the BeF2 molecule, the individual bond dipole moments do not cancel each other out. A water H2O molecule has a bond angle of 104.5o. An oxygen-hydrogen bond in a water molecule has an individual bond moment of 1.5 D. A water H2O molecule has a net dipole moment of 1.84 D.
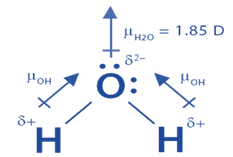
We are particularly interested in the symmetry of the dipoles. The dipoles in water point in the same direction, whereas in BeCl2, they point in opposing directions, which is why they cancel.
The dipole moment of NF3
Although there is a significant difference in electronegativity and electron affinity between nitrogen and fluorine atoms, NF3 has a relatively modest bond moment and a comparable structure to NH3.
The fact that the resultant bond moment of the three nitrogen-fluorine bonds acts in the opposite direction to that of the lone pair inserted at the nitrogen atom explains the low value of, NF3. However, in NH3, the generated bond moment acts in the same direction as the lone pair electrons.
Factors affecting dipole moment
Bond length
It increases as the bond length increases.
Polarity
The bigger the polarity difference, the greater the dipole value The magnitude of the charges (Q) is influenced by the polarity difference.
Geometry
If a molecule has opposing dipoles, it has no dipole moment (for example, BeCl2)
Chemical properties: Dipole moment
It is useful for determining certain chemical characteristics. It is, for instance, may affect the following attributes:
Solubility
The larger its dipole value, the greater the likelihood that the molecule will dissolve in a polar solution.
Melting/Boiling point
The boiling/melting point increases with increasing dipole moment.
Molecules having a higher dipole have stronger intermolecular forces. As a result, these forces are more difficult to break.
Stability/Reactivity
The larger the dipole value, the more reactive the system.
Uses of dipole moment
- It is used to determine the polarity of the connection. The polarity of the bond in a molecule rises as the amount of the dipole moment grows.
- It is used to determine a molecule’s structure or form. Because molecules with specified dipole moment values can be bent or angular in shape, they do not have a symmetrical structure. While molecules with zero dipole moment have a symmetrical structure or form.
- It is used to differentiate between cis- and trans-isomers. Trans-isomers have a higher value than cis-isomers, which have a lower dipole value.
- It is used to differentiate ortho, meta, and para isomers. Para-isomers often have dipole moments of zero, whereas ortho-isomers have dipole values larger than those of meta-isomers.
- The percentage ionic nature of covalent or ionic heteronuclear diatomic compounds is calculated using dipole data. Consider molecule AB, which has an observable dipole moment = μobs and a bond length of l cm.
- If the shared pair is located in the middle of the atoms, the connection is fully covalent, and the percentage ionic character is zero.
- However, if the connection is entirely ionic and B is more electronegative than A, A has a unit positive charge and B has a unit negative charge.
References
- https://unacademy.com/content/cbse-class-11/study-material/chemistry/dipole-moment/
- https://collegedunia.com/exams/dipole-moment-chemistry-articleid-528
- https://www.hellovaia.com/explanations/chemistry/physical-chemistry/dipole-moment/
- https://www.chemistrylearner.com/dipole-moment.html
