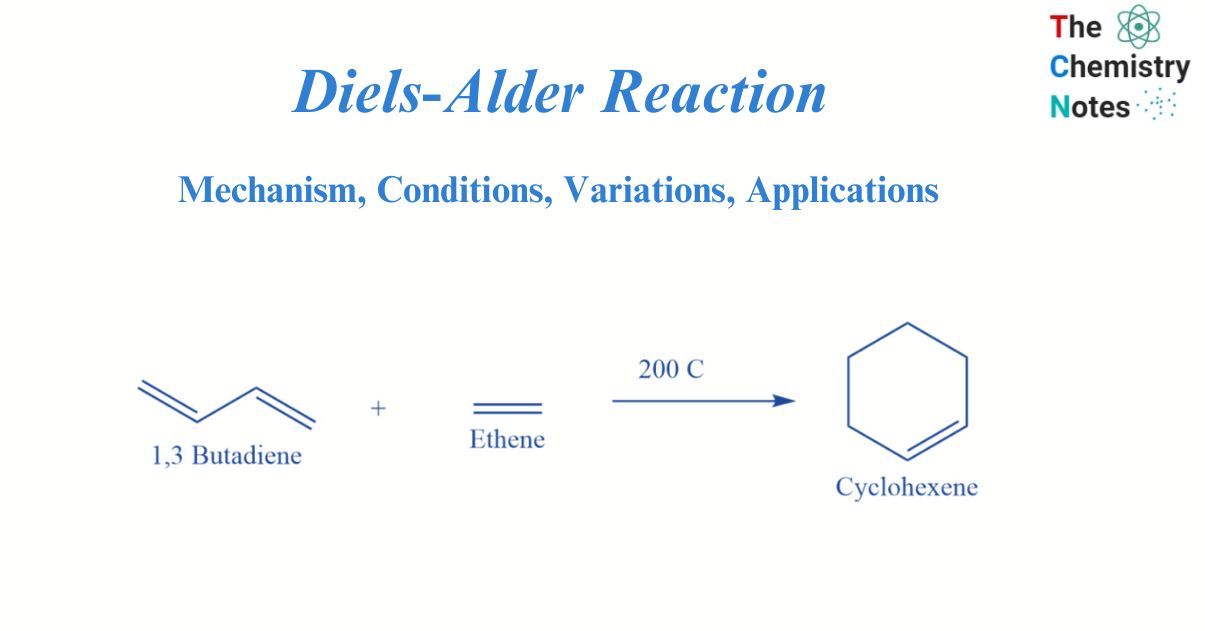
The Diels-Alder reaction is a pericyclic reaction between a conjugated diene (two double bonds) and a dienophile. This type of substituted alkene is known as a dienophile. This process produces a cyclohexene-substituted derivative.
The Diels-Alder reaction is an incredibly powerful reaction commonly utilized in synthetic organic chemistry due to its high degree of regio- and stereoselectivity (due to the concerted mechanism). Due to the conversion of two π-bonds into two new stronger σ-bonds, the reaction is frequently thermodynamically beneficial.
This reaction was discovered in 1928 by German chemists Otto Diels and Kurt Alder, for which they received the Nobel Prize in chemistry in 1950. Because two new carbon-carbon bonds are formed simultaneously, the Diels-Alder reaction can be employed to produce six-membered rings. The retro Diels Alder reaction is the opposite of the Diels Alder reaction, which creates a diene and dienophile from cyclohexane. It is possible to achieve this spontaneously using heat, acid, or base.
What is Diels-Alder’s reaction?
The Diels-Alder reaction is a chemical process that converts a conjugated diene and a dienophile to a cyclic olefin. This is a single-step reaction process in which bonds form and break at the same time, and the complete reaction occurs in one step in the presence of heat. Diels-Alder reactions are classified as cycloaddition reactions. The electrons are exchanged cyclically between the diene and the alkene to produce a cyclic adduct. Diels-Alder reactions are stereospecific. Dienophiles are electron-deficient species, whereas diene is electron-rich. As a result, the electron-donating group in the diene promotes the reaction, whereas the electron-drawing group on the Dienophiles accelerates it.
This is an important method for the preparation of alicyclic compounds. This reaction involves the reaction between diene and dienophile i.e., unsaturated compound. This reaction can be carried out in the presence or absence of the solvent or may be initiated by light, heat, or AlCl3 as a catalyst.
Electron-withdrawing groups like -COOH, -COOR, -NO2, -CN, and many others activate the dienophiles and activate the rate of reaction whereas, dienes are activated by electron donating groups like -CH3, and -OCH3.
Mechanism of Diels-Alder reaction
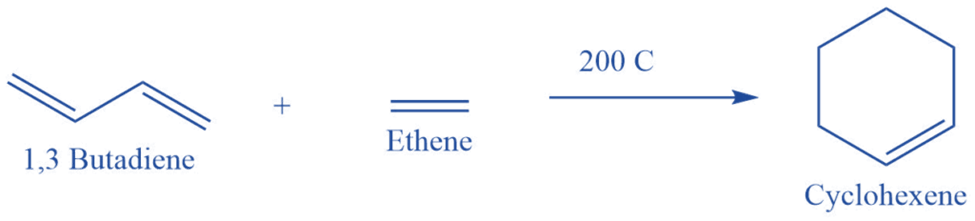
As the Diels-Alder reaction mechanism is concerted, the reaction takes place in a single-step cycloaddition. In this case, two unsaturated molecules interact to generate a cyclic adduct. There is an overall reduction in bond multiplicity. All bond forms and bond breaks occur at the same time.
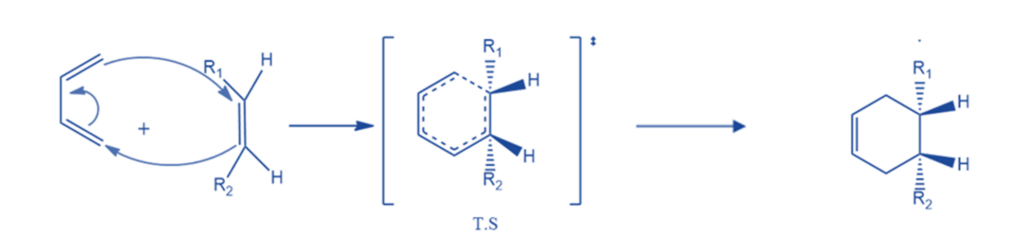
The Condition for Diels-Alder Reactions
Diels-Alder reaction conditions are as follows:
- The Diels-Alder reaction is allowed thermally, but not photochemically. This can be explained using the idea of orbital symmetry conservation and the Woodward- Hofmann principles.
- The dienes must also be in the s-cis conformation because if they are in the s-trans conformation, the dinophile cannot simultaneously access both ends of the diene.
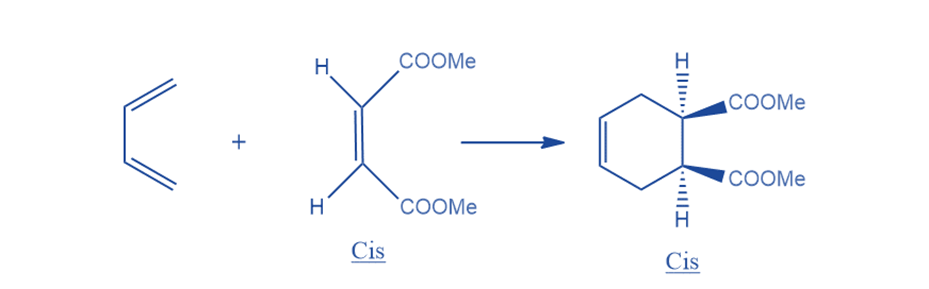
Stereochemistry of Diels-Alder reactions
The diels-alder reactions are stereoselective. This indicates that the two molecules reacting will produce a new molecule with a distinct stereochemistry. The Diels-Alder reaction is stereospecific in terms of both diene and dienophile. The stereochemistry of the diene and dienophile is preserved during the product formation. If the substituents of the dienophile are in cis-conformation, they will remain in the same cis-conformation in the product. Cis-orientation of a dienophile results in cis-product.
Similarly, substituents in the trans conformation stay trans in the product in dienophiles.
Variation of Diels-Alder reactions
Hetero-Diels-Alder process
The hetero-Diels-Alder process can be utilized to create six-membered heterocycles. The hetero-Diels-Alder reaction occurs when a diene reacts with a heteroalkene. The heteroalkene can be either substituted or unsubstituted. The hetero Diels-Alder variant can be used to create six-membered heterocycles containing one or more heteroatoms. The hetero Diels-Alder process is effective for generating heterocycles with nitrogen, oxygen, or sulfur atoms.
Using lewis acid
A Lewis acid is used as a catalyst in this variant. Aluminum chloride, boron trifluoride, and zinc chloride are common Lewis acids that can be utilized in these reactions. The Lewis acid increases the electrophilicity of the dienophile complex in these reactions. Increased reaction rates as well as enhanced stereo- and regioselectivity are benefits of this variant. These Diels-Alder processes can occur at low temperatures.
Asymmetric variation
Numerous variations of this reaction affect its stereoselectivity. The usage of a chiral auxiliary is one such example. Small-molecule organocatalysts can also be utilized to change the stereoselectivity of this process.
Applications of Diels-Alder reactions
- The Diels-Alder reaction is widely utilized in synthetic organic chemistry to form carbon-carbon bonds. It is particularly valuable for making six-membered rings.
- The Diels-Alder reaction is a prominent transformation used by organic chemists to efficiently produce molecular complexity. It is employed in the synthesis of pharmacologically active compounds, agrochemicals, and flavors and fragrances in industrial applications.
- It is utilized in the production of synthetic steroids such as cortisol and vitamin D.
- The Diels-Alder process is used for producing rubber and plastic. It is also used in pharmaceuticals and biomedical engineering.
References
Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
