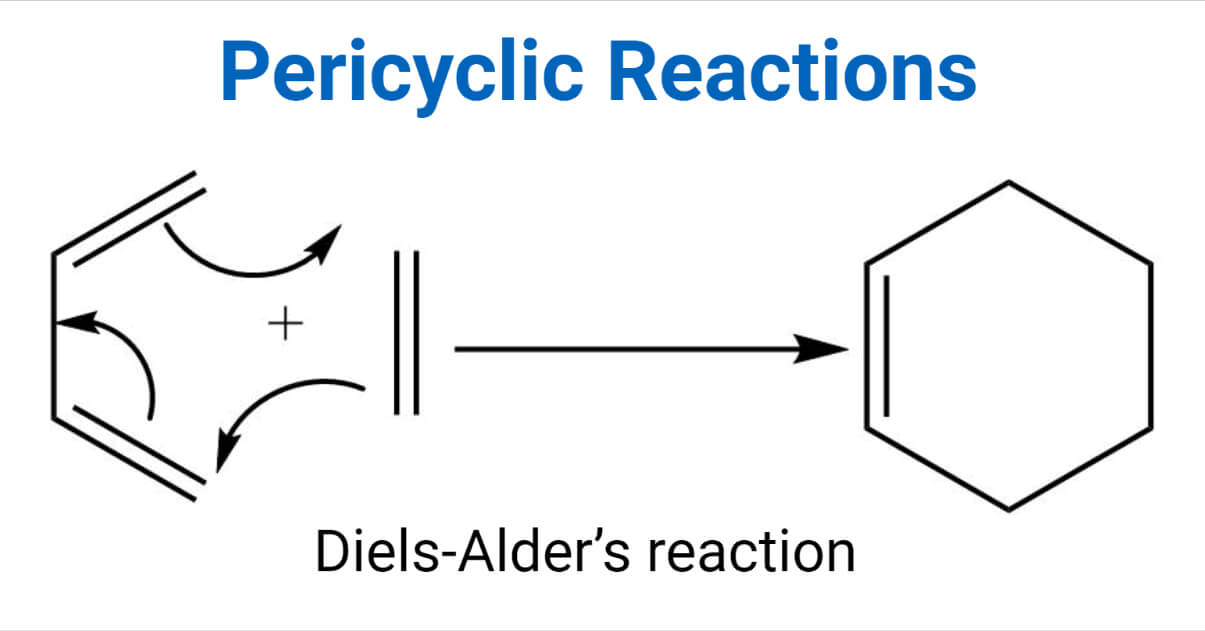
Pericyclic reactions are concentrated reactions that proceed through the cyclic transition state. It requires light or heat and is highly stereospecific.
Pericyclic reactions involve the cyclic rearrangement of π or σ electrons in a single concerted step. Since the electronic movement is cyclic pericyclic reactions cannot be described in electrophilic-nucleophilic interaction.
e.g, Diels-Alder’s reaction.
Interesting Science Videos
Characteristics of pericyclic reactions
1. Pericyclic reactions are not affected by radical initiators, Scavenging reagents or electrophilic or nucleophilic catalysts, or solvent changes.
2. They proceed by the concerted series of bond formation and bond-breaking processes and are highly stereospecific.
3. Ionic, free radicals, or other intermediates are not involved in the reaction path of pericyclic reactions.
Pericyclic reactions involve the reorganization of bonding electrons through the cyclic transition state. All these reactions have the potential to be reversed. For instance, Cycloreversion is the inverse of cycloaddition and involves the ring cleavage that involves the conversion of two sigma bonds to two pi bonds.
Terms involved in the pericyclic reactions
Pericyclic reactions involves various terms they are:
Suprafacial
Suprafacial is the process in which bonds are formed or broken on the same face of the system undergoing a reaction. It corresponds to the involvement of both the interior or exterior lobe of a bond.
Antrafacial
Antrafacial is the process in which bonds are formed or broken on the opposite face of the system undergoing a reaction. It corresponds to the involvement of one interior and one exterior lobe of a bond.
Conrotatory
The process in which the terminal 2p π orbitals are rotated in the same direction.
Disrotatory
The process in which the terminal 2p π orbitals are rotated in the opposite direction.
Theories involved in the pericyclic reaction
The three different types of theories involved in the pericyclic reactions are:
1. Woodward Hoffmann Rule
2. Frontier Molecular Orbital Theory
3. Huckel Mobies theory
Woodward Hoffmann rule
In 1965, Robert Woodward and Roald Hoffmann proposed a set of rules for predicting the outcome of pericyclic reactions. The theory of pericyclic reaction is based on the conservation of orbital symmetry. The combination involving p-orbitals to form the sigma bond occurs by the overlap of like sign orbitals i.e. +ve lobe overlaps with another +ve love or a -ve lobe overlap with another -ve lobe. However, the mode of overlap achieved depends on the number of π electrons involved in the reactions and whether the reaction is under thermal or photochemical control.
General rules to predict the stereochemistry of pericyclic reactions
1. Pericyclic reaction can occur only if the symmetries of reactant and products molecular orbitals are the same.
2. Pericyclic reactions are said to be symmetry-allowed if the symmetries of the reactant and product orbitals match up or “correlate.” The reaction is symmetry-disallowed if the symmetries of the reactant and product orbitals do not correlate.
Correlation diagram
A correlation diagram is an orbital correlation diagram that is created by connecting the energy-ordered orbitals of reactants to those of products using symmetry elements preserved throughout the reaction.
The correlation diagram is based on two fundamental rules they are:
Rule 1: Only the orbital of the same symmetry can interact
It means symmetry (S) orbitals in the reactants must transform into symmetry (S) orbitals in the product, and antisymmetry (A) orbitals in the reactants must transform into antisymmetry (A) orbitals in the product.
Rule 2: Non-crossing rule
It states that the orbitals of the same symmetry do not cross. The non-crossing rule does not apply to orbitals of different symmetries. Hence the correlation lines representing orbitals of different symmetry may cross.
Conrotatory interconversion of butadiene and cyclobutene and vice versa
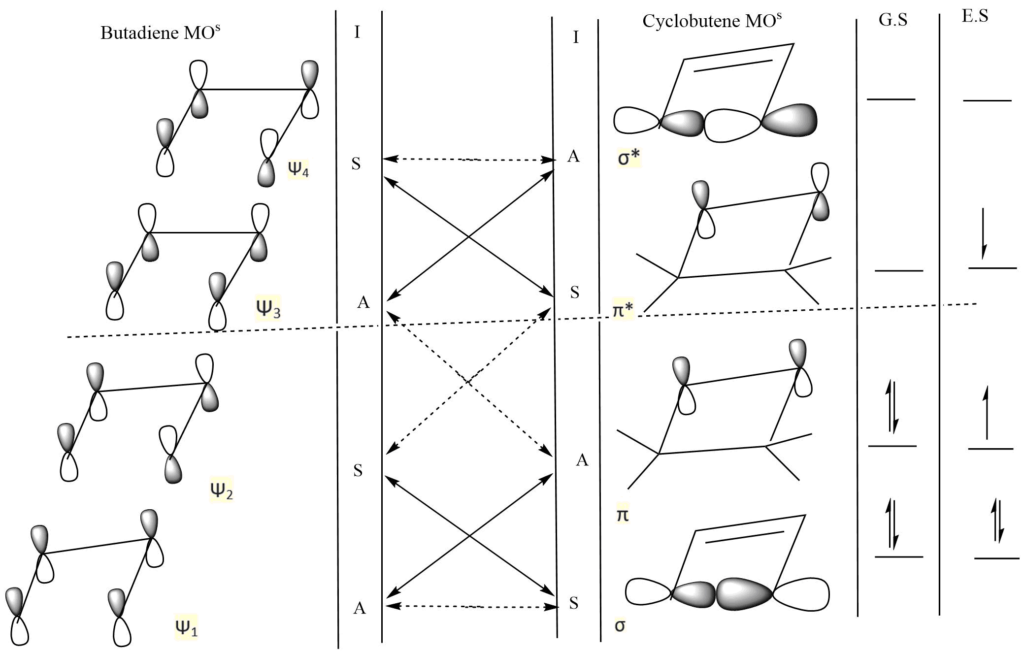
In the above reaction, I indicate conrotatory interconversion, while the solid line and the dotted line indicate allowed symmetry and forbidden symmetry, respectively.
For the ground state process
Ψ21 Ψ22 (butadiene) ⇿ σ2 π2 (cyclobutene)
There is no energy barrier. It is a symmetry-allowed process. So the interconversion is favorable.
σ2 π2 (cyclobutene) ⇿ Ψ21 Ψ22 (butadiene)
There is no energy barrier. It is a symmetry-allowed process. So the interconversion is favorable.
Therefore thermal and conrotatory conversion of butadiene to cyclobutene and vice versa is a symmetry-allowed process.
For the excited state process
Ψ21 Ψ2 Ψ*3(butadiene) ⇿ σ π2 σ* (cyclobutene)
There is a large energy barrier. It is symmetry forbidden process. So the interconversion is not favorable.
σ2 π π* (cyclobutene) ⇿ Ψ21 Ψ2 Ψ4* (butadiene)
There is a large energy barrier. It is symmetry forbidden process. So the interconversion is not favorable.
Therefore photochemical and conrotatory conversion of butadiene to cyclobutene and vice versa is symmetry forbidden process.
The conversion of butadiene to cyclobutene and vice versa is thermally allowed by the conrotatory process and vice versa.
Frontier molecular orbital (FMO) concept
According to the FMO concept, the highest occupied molecular orbital (HOMO) of one molecule reacts with the lowest unoccupied molecular orbital (LUMO) of the second molecule, and stabilization occur as they approach each other. It is the methodology for predicting whether the given pericyclic reaction is allowed by examing the symmetry of HOMO (in case of unimolecular reaction) and LUMO (if the reaction is bimolecular) of the second partner.
When the HOMO of one reactant has the same symmetry as the LUMO of the other, a pericyclic reaction is allowed.
General rules for (FMO) concept
- The number of atomic orbitals must equal the number of molecular orbitals.
- The lowest energy molecular orbital lacks a nodal plane.
- As the number of nodal planes increases, the relative energy of molecular orbitals also increases.
- Two electrons occupy the molecular orbitals, beginning with the energetically lowest molecular orbitals.
Huckel-Mobius theory
It is a feasible alternative to the Woodward-Hoffmann rules. According to this theory, pericycle reactions involving 4n+2 electrons are thermally allowed, while pericycle reactions involving 4n electrons are photochemically allowed.
General rules for Huckel-Mobius theory
1. 4n+2 (n= integer number) π electrons are thermally aromatic and stable.
2. 4n π electrons are photochemically aromatic and stable.
3. 3. 4n π electrons have lower thermal stability and are antiaromatic.
4. 4n+2 π electrons are photochemically antiaromatic and less stable.
Types of pericyclic reactions
There are four different types of pericyclic reactions they are:
1. Electrocyclic reaction
2. Cycloaddition reaction
3. Sigmatropic reaction
4. Cheletropic reaction
Electrocyclic reaction
This reaction involves the concerted cyclization of the n π -electron system to the (n-2) π + 2 σ electron system.
Reaction:

A Single-molecule can undergo electrocyclic ring opening and electrocyclic ring-closing reaction by converting σ bond to π bond and vice versa. Photochemical electrocyclic reactions occur in a conrotatory manner for a conjugated system with an odd number of bonds. In contrast, for an even number of π bonds, it takes through a disrotatory manner.
Rules for electrocyclic reaction
Woodward selection rules for the electrocyclic reaction in the correlation table allowed only:
| System | No. of electrons involved | Disrotatory | Conrotatory |
| Mobius | 4n | Photochemically allowed | Thermally allowed |
| Huckel system | 4n+2 | Thermally allowed | Photochemically allowed |
According to the Woodward-Hoffman rule reaction involving 4n π electrons undergoes a disrotatory ring opening in photochemical conditions, while in the thermal condition, it undergoes a conrotatory ring opening. In a disrotatory ring opening, the stereochemistry is preserved, whereas in a conrotatory ring opening, the stereochemistry is altered.
Cycloaddition reaction
A cycloaddition reaction is a concerted combination of two π -electron systems to form a ring of atoms with two new σ bonds. It involves the addition of m π electrons to the system of n π electrons to form a new ring system with the formation of two new σ bonds. Such reactions are also called m+n cycloaddition reactions. Depending on the number of components involved, it can be termed an (m+n+….) cycloaddition reaction.
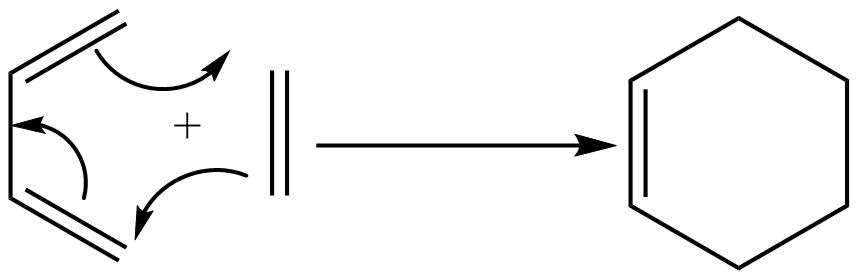
Classification of cycloaddition reaction
Cycloaddition reactions are classified into different types based on three factors, they are:
1. The orbitals undergoing modification (σ or π).
2. The number of electrons present on each component involved in the cycloaddition reaction.3. The stereochemistry of cycloaddition reaction (antrafacial or suprafacial)
Example:
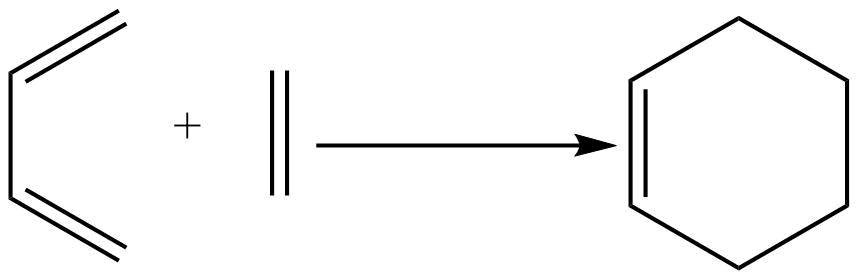
Rules for the cycloaddition reaction
| No. of participating electrons (m+n) | Allowed in ground state/Forbidden in an excited state (thermally) | Allowed in excited state/Forbidden in a ground state (Photochemically) |
| 4q | ms+ na ma+ ns | ms+ ns ma+ na* |
| 4q+2 | ms+ ns ma+ na* | ms+ na ma+ ns |
Sigmatropic reaction
In a sigmatropic reaction, σ- bond shifts to a new position with a corresponding reorganization of the π-bonds. In this reaction, either one or both ends of the bond may move, and the π system is reorganized. The notation (i, j) is used to express the order of the sigmatropic reaction. The new bond could be formed on the same side or the opposite face of the π framework. These processes are termed suprafacial and antrafacial, respectively.
![[3,3] sigmatropic shift](https://scienceinfo.com/wp-content/uploads/2022/08/sigmatropic-reaction-1024x183.png)
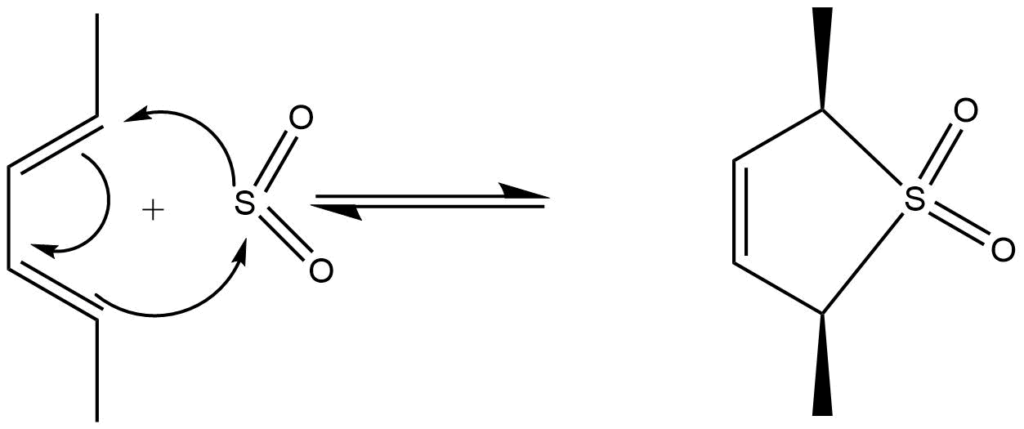
Cheletropic reaction
These are the cyclization reactions in which one of the components interacts through a single atom or in which both termini of the π -system are linked by the same atom.
e.g: Addition of SO2 to dienes
References
- Charles H. Dupuy and Orville L. Chopman, ” Molecular reactions and photochemistry”, Prientice-Hall, 1975.
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/pericycl.htm.
- https://www.studocu.com/row/document/government-college-university-faisalabad/oragnic-chemistry-1/pericyclic-reactions-and-theories/9013026.
- https://byjus.com/chemistry/pericyclic-reactions/.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Pericyclic_Reactions/Woodward_Hoffmann_rules
- https://www1.udel.edu/chem/mpwatson/mpwatson/Chem_633_files/Pericyclic_Reactions_handout%20for%20lecture.pdf.
- http://diposit.ub.edu/dspace/bitstream/2445/61063/22/2.%20Organic%20Synthesis.%20Pericyclic%20Reactions.pdf.
- https://www.scienceabc.com/pure-sciences/what-are-pericyclic-reactions.html.
- https://www.alchemyst.co.uk/pdf/Organic/pericyclics.pdf.
