Cyclic compounds are organic compounds in which carbon atoms are arranged to form rings. They are also known as closed chain compounds. Cyclic compounds in which the closed ring is entirely made up of carbon are carbocyclic compounds. They are also known as homocyclic compounds. Homocyclic compounds are further classified into two types. They are:
- Alicyclic
- Aromatic
Alicyclic compounds
They are cyclic compounds in which the carbon atoms are bonded together to form one or more rings. They do not have aromatic characteristics. They could either be saturated or unsaturated. Like an open chain compounds they can be further classified into cycloalkane, cycloalkene and cycloalkyne.

Aromatic compounds
Aromatic compounds are cyclic compounds that contain at least one aromatic ring. The aromatic ring is a highly stable planar ring of atoms with resonance structures that include both alternating double and single bonds.
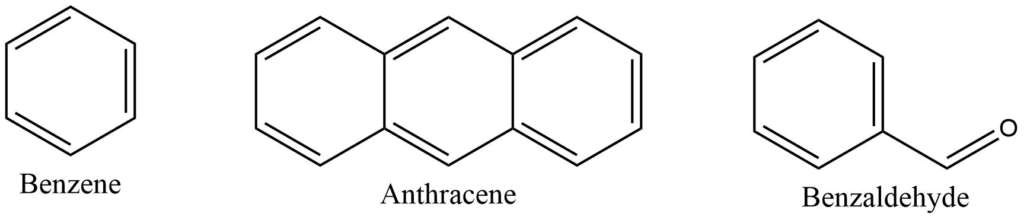
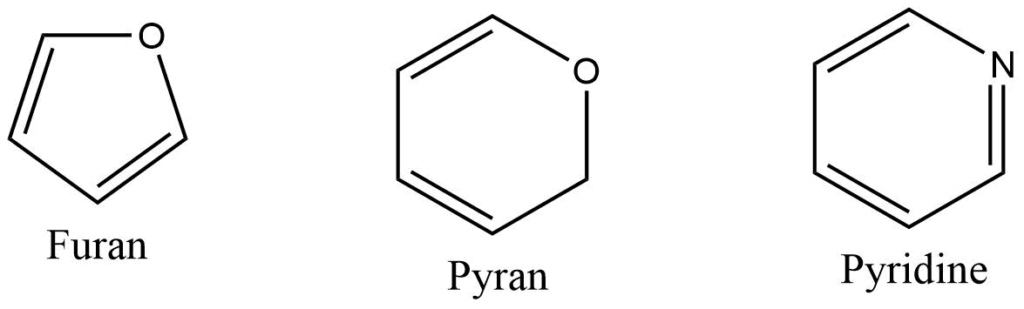
Heterocyclic compounds
Heterocyclic compounds consist of a carbon chain along with other element like oxygen, sulphur, nitrogen and others.
Nomenclature of cyclic compounds
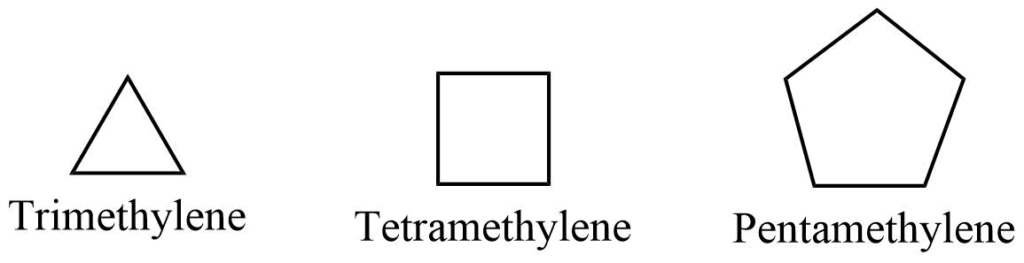
a. Common system
The saturated alicyclic hydrocarbon can be referred to as polymethylene since it contains several methylene groups that are linked together to form a cyclic structure. The number of methylene groups is indicated by a Greek or Latin prefix, such as tri, tetra, penta, hexa, and hepta, for 3, 4, 5, 6 , and 7 methylene groups, respectively.
b. IUPAC system
According to the IUPAC nomenclature, saturated monocyclic compounds are referred to as cycloalkanes. This name is obtained by prefixing the name of the corresponding open-chain hydrocarbon with the cyclo prefix.

When there is only one substituent in a substituted cycloalkane, the name of the substituent is used as a prefix before the parent name.
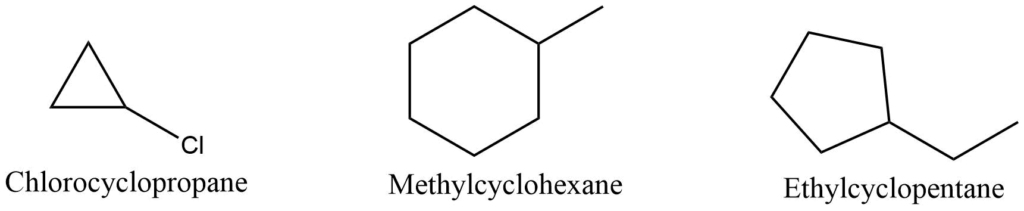
If the molecule contains two or more substituents, the substituent is prefixed before the name in alphabetical order. The position of the substituent is represented by the number of the carbon atom to which it is connected. Ring carbon is numbered by the lowest sum rule to identify the position of the substituent.

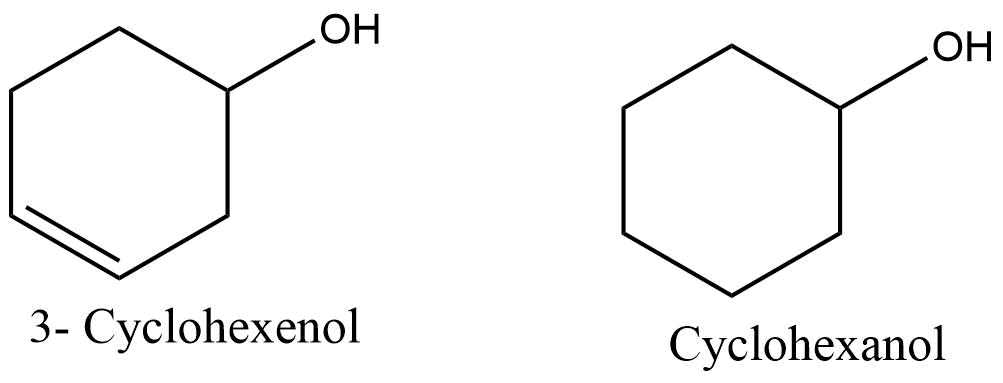
In the case of unsaturated monocyclic hydrocarbon, the rule is applied to an alkene or alkyne, i.e, they are named cycloalkene or cycloalkyne. But the doubly triply bonded carbons are considered to occupy the position 1 and 2.

In the case of functional groups, the functional group takes priority over ‘ene’ and ‘yne’ and appears last in the name. For illustration:
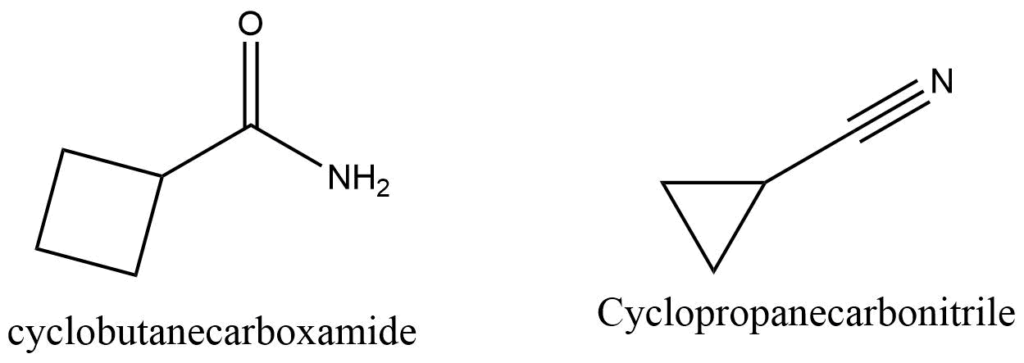
Aldehydes, carboxylic acids, amides, nitriles, and other functional groups cannot be included in the base c name of the cyclic part of the molecule; instead they represent a branch from it. For example,
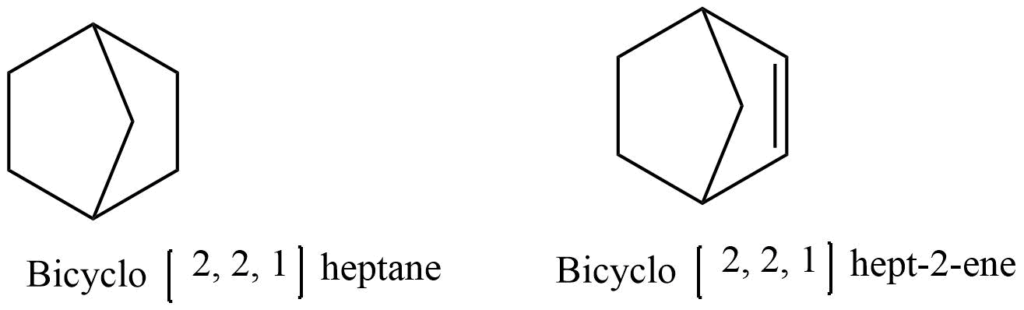
Bicyclo compounds
A compound containing two rings with two or more common carbon atoms is known as a bicyclic compound.The prefix “bicyclo” is added to the name of the parent hydrocarbon to give them their names. By counting all of the carbon atoms present in the molecule, the name of the parent hydrocarbon can be determined. In parentheses, the number of carbon atoms in each bridge connecting the two tertiary carbon atoms is listed in decreasing order.
Industrial source
The alicyclic compounds, particularly those with five and six members, are discovered to be particularly abundant in the naphtha fraction of petroleum, which comes from a particular region, namely California. For this reason, the alicyclic compounds are also known as naphthenes.
Cyclohexane, methylcyclohexane, methyl cyclopentane, and 1,2 di methylcyclohexane are found in the naphtha fraction of petroleum. These cycloalkanes can be converted into cycloalkene by catalytic elimination of hydrogen.
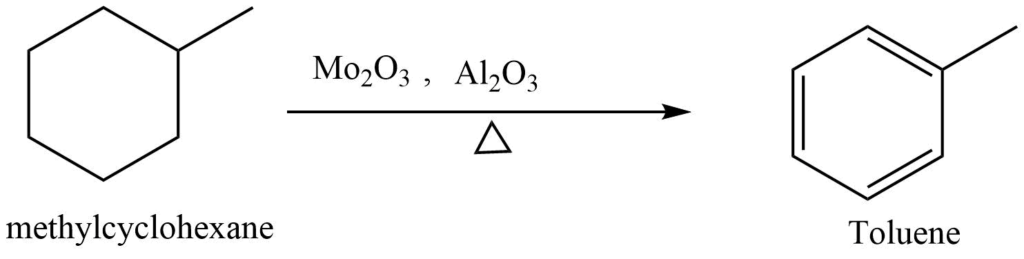
Similarly, the addition of hydrogen to aromatic compounds yields cyclic aliphatic compounds.
Preparation of alicyclic compounds
Preparation of alicyclic compounds from the open chain compounds mainly involves two steps
- The conversion of the open chain compound into the cyclic compound. This step is called cyclization.
- Conversion of cyclic compounds into other required compounds. For example, Conversion of cycloalkane into cycloalkene.
I. From dihalides (Freund’s method)
Terminal dihalides undergo cyclization reaction on treatment with sodium or zinc to produce corresponding cycloalkanes. Terminal halogen derivatives in which the hydrogen is farther apart than the 1-6 position do not undergo cyclization reaction but undergo the wurtz reaction.
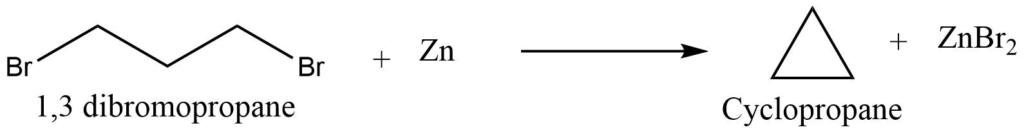
II. From salt of dicarboxylic acids (Wisliceus method)
Distillation of the calcium thorium or barium salt of a dicarboxylic acid, such as adipic acid, pimelic acid, or suberic acid, produces cyclic ketone. This cyclic ketone can then be reduced by Clemmensen reduction to produce cycloalkane.

III. From Diels-Alder reaction
This is an important method for the preparation of alicyclic compounds. This reaction involves the reaction between diene and dienophile i.e., unsaturated compound. This reaction can be carried out in the presence or absence of the solvent or may be initiated by light, heat or AlCl3 as catalyst.
Electron withdrawing group like -COOH, -COOR, -NO2, -CN and many others activate the dienophiles and activate the rate of reaction whereas, dienes are activated by electron donating groups like -CH3, and -OCH3.
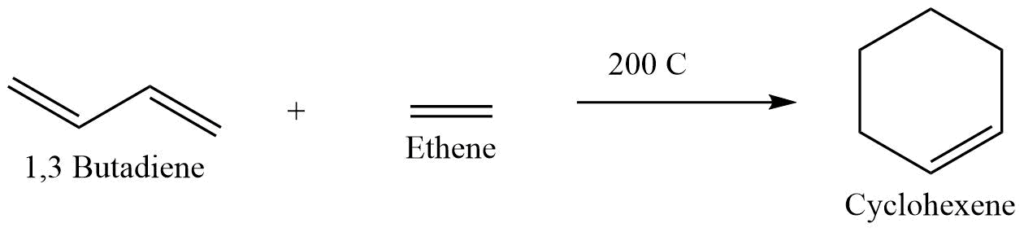
IV. From esters of dicarboxylic acids (Dieckmann’s method)
An important approach for the synthesis of five, six, and seven members alicyclic compounds is provided by Dieckmann’s method i.e., an intermolecular claisen condensation reaction.
On reaction with sodium metal or sodium ethoxide, the ester of a suitable carboxylic acid undergoes cyclization to produce 2-carboalkoxycycloalkanone, which upon hydrolysis yields 2-carboxycycloalkanone. Further decarboxylation of 2-carboxycycloalkanone produces cycloalkanone, which is reduced to form cycloalkane.
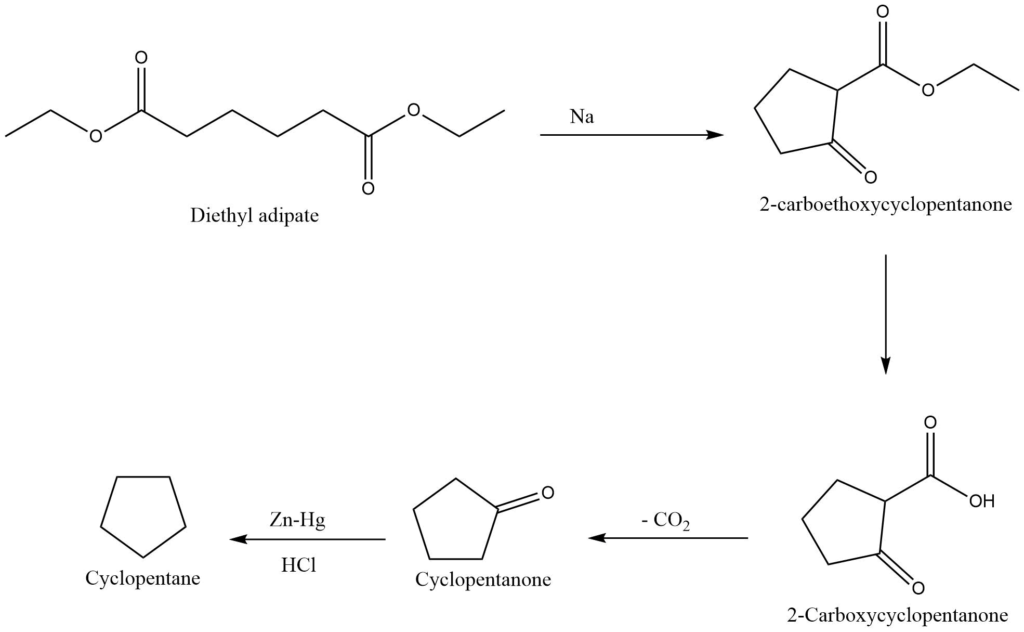
Reactions of cyclic compounds
Alicyclic compounds shows similar types of reaction as open chain compounds with few exceptions.
I. Free radical susbstitution reactions
Cycloalkane mainly undergoes a free radical substitution reaction like open chain compounds. For example Cyclopropane reacts with chlorine to give chloropropane

I I . Addition reaction
Cycloalkene readily undergoes an addition reaction, whereas cycloalkane is inert towards the addition reaction.
a. Recation with halogen
Cycloalkene undergoes an addition reaction with halogen to give a halogenated derivative of cycloalkane. For example, the Reaction of cyclohexene with bromine produces 1,2 dibromo cyclohexane
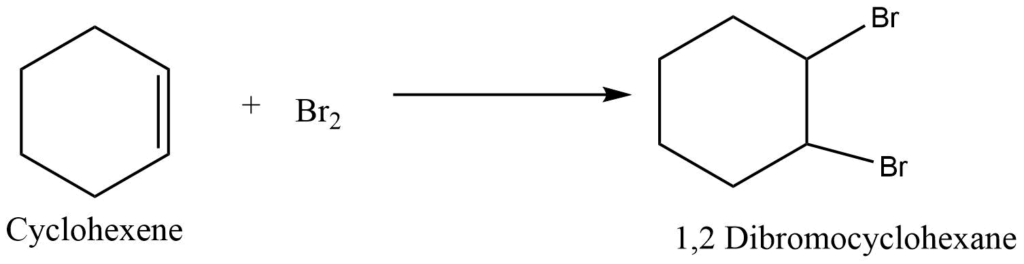
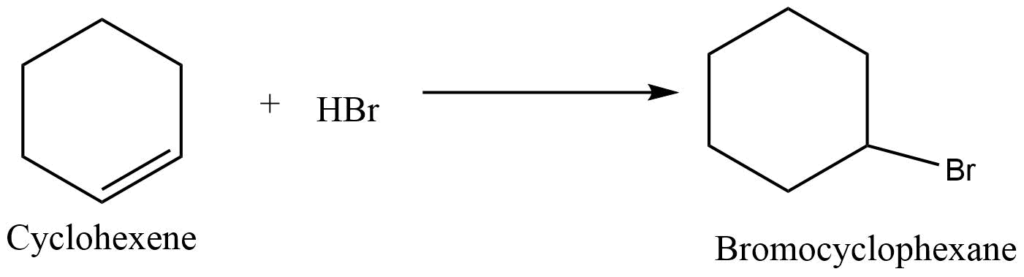
b. Reaction with hydrogen halides
Cycloalkene reacts with hydrogen halide to give monosubstituted derivative of cycloalkane.
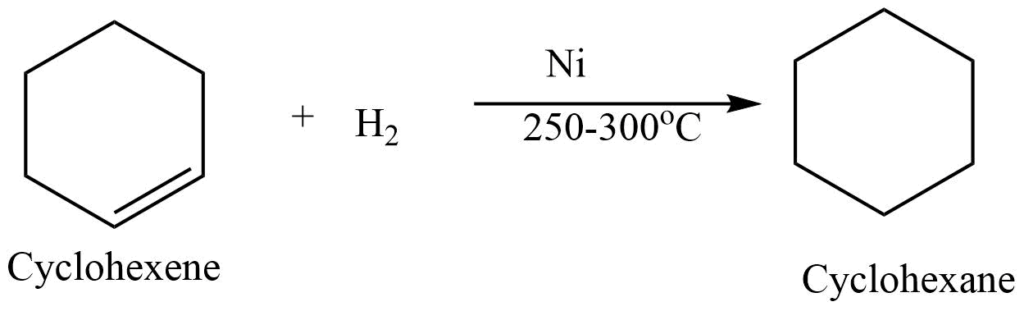
III. Hydrogenation reaction
Catalytic hydrogenation of cycloalkene produces cycloalkane. For example, hydrogenation of cyclohexene produces cycloalkane
References
- Bahl A. & Bahl B. S. (2006). A textbook of organic chemistry (for b. sc. students) (18th rev. & enlarged ed. 1st multicolour illustrative). S. Chand.
- Smith M. & March J. (2020). March’s advanced organic chemistry : reactions mechanisms and structure (Eighth). John Wiley & Sons.
- https://www.yaclass.in/p/science-state-board/class-10/carbon-and-its-compounds-13907/carbon-compounds-types-properties-nomenclature-11669/re-6d86ba5c-c025-4aca-a7a6-da496f5f7258.
- https://www.vedantu.com/chemistry/alicyclic-compound.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alkanes/Nomenclature_of_Alkanes/Nomenclature_of_Cycloalkanes.
- https://byjus.com/chemistry/cycloalkanes/
