Coumarin is a naturally occurring substance belonging to the benzopyrone family. It is also known as 1-benzopyran-2-one. Vogel identified the first parent coumarin from tonka bean (Dipteryx odorata) in 1820. The name coumarin is derived from the French word “Coumarou” for tonka bean.
A naturally occurring volatile active molecule called coumarin can be discovered in a broad spectrum of plants. Additionally, food plants like strawberries and cherries contain coumarin. Deer tongue, mullein, Ceylon cinnamon, Cassia cinnamon, and various Prunus species of cherry blossom trees are other plants that contain substantial amounts of coumarin.
Simple coumarins, furanocoumarins, pyrano coumarins (linear and angular type), dihydrofurano coumarins, phenyl coumarins, and bicoumarins are the six basic classes of natural coumarins.
It is estimated that around 1300 natural coumarins isolated from plants, fungi, and bacteria are currently known. Coumarins are secondary metabolites that typically perform a protective role in suppressing many biological processes in nature.
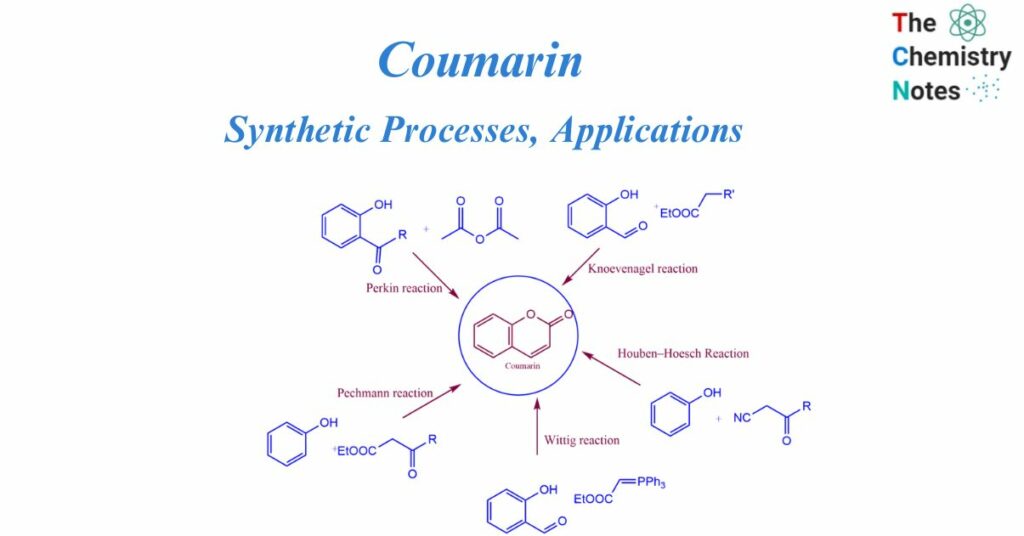
Coumarin synthesis
Perkin described the coumarin synthesis for the first time in 1868. Heating sodium salt of salicylaldehyde with acetic anhydride resulted in the reaction. Further investigation into this procedure resulted in the broad synthesis of cinnamic acid and its analogs, which is known as the Perkin reaction. The Perkin process, von Pechmann condensation, Knoevenagel condensation, Baylis-Hillman reaction, Michael addition, Kostanecki reaction, and Heck lactonization reaction are all common one-step procedures for the synthesis of coumarin derivatives.
Methods of Coumarin synthesis
Perkin reaction
It involves the reaction between salicylaldehyde and acetic anhydride react to generate coumarin.
Pechmann reaction
The Pechmann reaction involves the condensation of different phenols with β-ketoesters in the presence of homogeneous catalysts such as trifluoroacetic acid or Lewis acids (e.g., AlCl3).
Knoevenagel reaction
The Knoevenagel reaction includes the condensation of different o-hydroxy aldehydes with active methylene compounds in the presence of a base catalyst.
Houben–Hoesch
Houben–Hoesch reaction is a condensation reaction. It involves the reaction of β-keto nitriles and phenol derivatives to form Comarin.
Wittig reaction
Coumarin derivatives are generated as a result of the Wittig reaction. This reaction involves the reaction between aromatic aldehydes or ketones with a phosphonate or phosphorous ylide.
Other Methods of Coumarin Synthesis
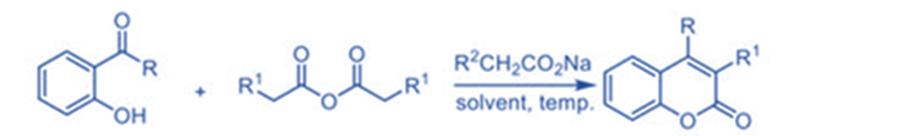
Kostanecki-Robinson
The Kostanecki-Robinson coupling reaction could be used to create coumarin derivatives. The reaction of aliphatic anhydride with aryl ketone with a hydroxyl group substitution generates the desired product as coumarin with good yields.
Heck-Lactonization reaction
The Heck-Lactonization reaction can be used to synthesize coumarin analogs using Pd catalysis, and several reaction conditions were explored utilizing aqueous solutions and organic solvents.
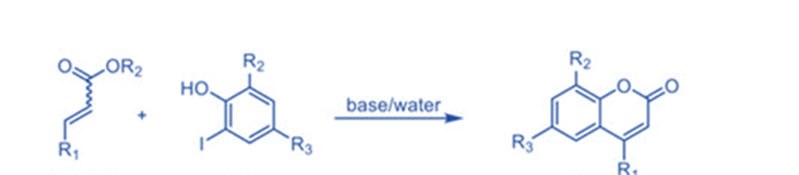
Coumarin synthesis by intermolecular hydroarylation of alkene
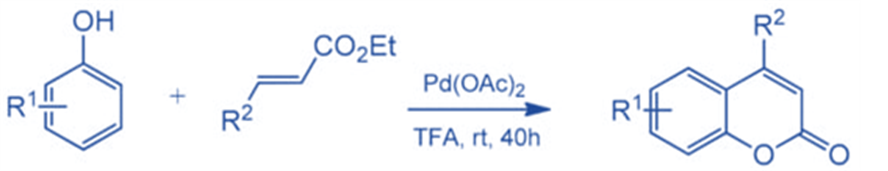
Direct C-H bond alkenylation is another appealing technique for coumarin synthesis, and some successful instances have been established, including Pd-catalyzed arylation of acrylates. Kitamura and colleagues published the first palladium-catalyzed reaction in 2005.
Several phenols were reacted with ethyl cinnamate, ethyl crotonate, and ethyl acrylate to generate the respective compounds in moderate to good yields. Under the reaction circumstances, the oxidative coupling of electron-rich phenols was a competitive reaction, resulting in lower product yields.
Application of Coumarin
- Coumarins can also act as selective enzyme inhibitors, interacting with targets in the therapy of disorders including Parkinson’s and Alzheimer’s.
- Coumarins have a remarkable range of applications in the biomedical sciences, medical research, and many other industries, including perfumery.
- These phytochemicals have antibacterial (agasyllin, felamidin, and armillarisin A) and anti-inflammatory (coumarin and esculetin) activities, as well as anticancer, antiviral, antioxidant (Esculetin), and antifungal characteristics.
References
- https://www.organic-chemistry.org/synthesis/heterocycles/benzo-fused/coumarins.shtm.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7022947/.
- https://byjus.com/chemistry/coumarin-synthesis/
- https://www.intechopen.com/chapters/84910
