Combinatorial synthesis is a synthesis technique that allows for the generation of a large number of related molecules at the same time. These collections are known as libraries, and they can be employed in any discovery effort that requires high-throughput analysis.
Combinatorial synthesis employs technologies (solid phase synthesis) and strategies (hundreds or thousands of simultaneous reactions) that enable the rapid creation of large, organized collections of organic molecules, known as molecular libraries. Deconvolution enables the assessment of mixtures for biological activity without the separation or purification of individual components. If carefully constructed, these libraries act as key resources for the identification and generation of novel compounds for pharmacology.
What is combinatorial synthesis?
Combinatorial synthesis is a set of procedures that allows for the simultaneous synthesis of numerous molecules. It is one of the significant new approaches created by pharmaceutical industry researchers to lower the time and costs associated with developing effective and competitive new medications.
Combinatorial chemistry can be used to generate enormous libraries of thousands of chemicals regularly. The parallel advancements in automated technologies that enable chemists to prepare libraries more effectively have benefited advances in the design, synthesis, purification, and analysis of huge arrays of chemicals.
Principle of combinatorial synthesis
Instead of synthesizing compounds one at a time, the fundamental concept of combinatorial chemistry is to produce a large number of distinct compounds at the same time and then identify the most promising compound for further development through high throughput screening.
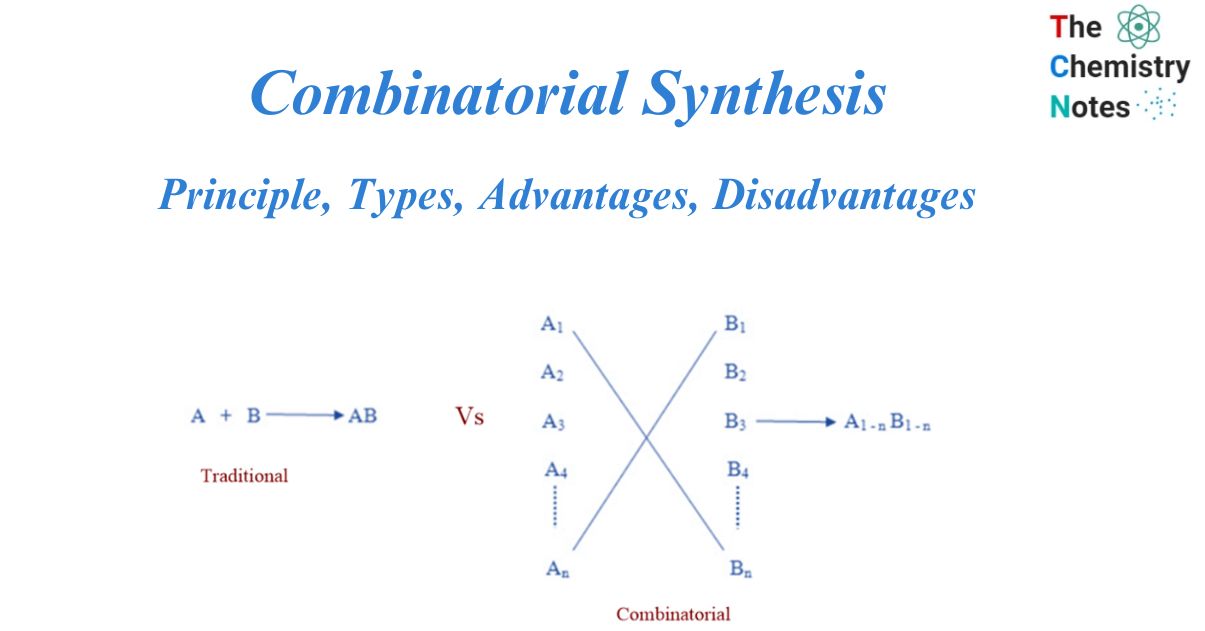
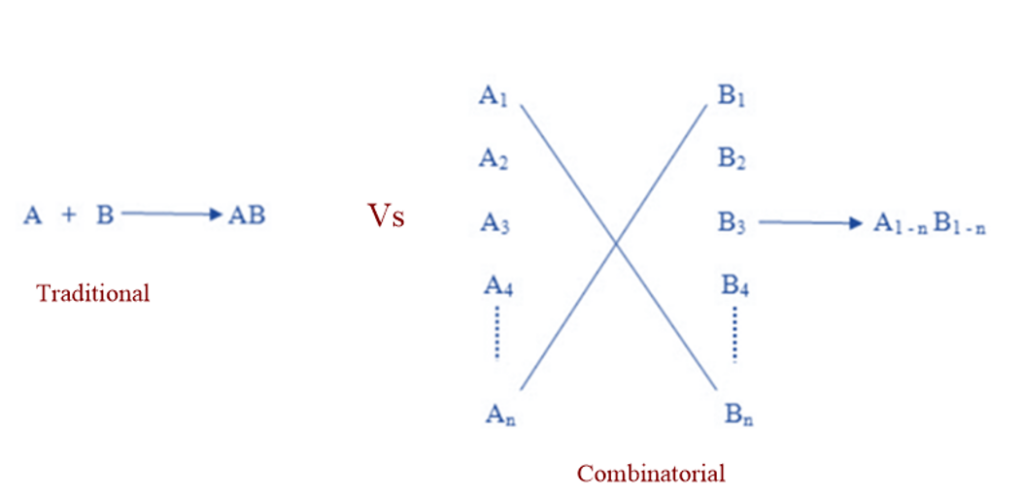
Traditional VS Combinatorial synthesis
Potential lead compounds were traditionally synthesized one at a time. This compound’s biological activity was tested, and the results would be incorporated into the next step of design. This conventional method was useful, but it was time-consuming and costly. Computational chemistry enabled more rational compound design for testing, while high throughput screening enabled rapid in vitro experiments. Synthesis of one compound at a time could no longer keep up, and so became the process’s rate-limiting step. The solution to this dilemma was combinatorial chemistry. Instead of doing many A x B type reactions, the combinatorial technique allows for the coverage of many An x Bn possibilities in a single reaction.
Combinatorial procedures are extremely diverse, and these products could be created individually or in mixes, utilizing either solution or solid phase processes. Whatever technique is utilized, the common denominator is that productivity has increased beyond the levels seen in the previous century.
With the introduction of combinatorial approaches, the development of new processes for the generation of collections of structurally related compounds (libraries) has renewed random screening as a paradigm for drug discovery, raising enormous excitement about the possibility of finding new and valuable drugs in a short time and at reasonable costs.
General techniques for the combinatorial synthesis
Solid phase synthesis
This method involves binding reactants to a polymeric surface and modifying them while they’re still there. After the synthesis, the final product is released. Solid phase chemistry underpins combinatorial synthesis. Filtration is used as a separation and purification process. The heart of library synthesis is the straightforward elimination of undesirable substances by filtration.
Substituted resin beads are utilized as a solid phase in this synthesis, which is essentially a gel-like matrix of linked polymeric molecules distended by solvent molecules.
Advantages
- Reactants can be linked to certain beads.
- Beads can be combined and reacted in the same reaction vessel. The products generated are unique to each bead and physically distinct.
- Excess reagents and by-products can be conveniently eliminated.
- Since the reaction intermediates are bound to the bead, they do not need to be separated and purified.
- Individual beads can be separated to isolate specific products.
- After cleaving the product, the polymeric support can be regenerated and re-used.
Parallel synthesis
This technique is typically used for generating combinatorial libraries made up of individual chemicals. It is not appropriate for creating libraries containing thousands to millions of compounds. Parallel synthesis involves the preparation of chemicals in different reaction vessels at the same time, i.e. in parallel.
Individual reaction vessel arrays are frequently in the shape of a grid of wells in a plastic plate or a grid of plastic rods called pins attached to a plastic base plate that fits into a corresponding set of wells. The process takes place on so-called plastic ‘crowns’ placed onto the tops of the pins, with the building blocks attached to these crowns by linkers similar to those found on resin beads.
In general, both well and pin arrays are employed; the position of each synthetic pathway in the array, and hence the structure of the product of that pathway, is usually designated by a grid code.
Advantages
- It generates the compounds one at a time in its vessel. As a result, the product’s identification is already known.
- There is no need for deconvolution.
- Each chemical is largely pure in its region.
- A compound’s structure is determined by its defined position.
- Simpler biological analysis
Mixed combinatorial synthesis
It is a common synthetic technique for producing a wide range of various analogs, with each reaction vessel or tube containing a combination of products. The identity of the structures in each vessel during this procedure remains uncertain.
It can quickly synthesize a huge number of chemicals and is effective for finding a lead compound. Using this method, active combinations are checked for activity, and inactive mixtures are stored for combinatorial synthesis.
The active combinations are investigated further in order to find active components.
Encoding of combinatorial libraries
The number of libraries with “desirable properties” is reduced by the screening process. It is now critical to discover the identity of the “winning” library member. Encoding is the technique of identifying the active ingredient in a mixture of chemicals.
Identification of active components involves the following steps:
Positional encoding or deconvolution
Resynthesis and rescreening are used in this procedure to determine the identity of the active molecule. In other words, it is the process of optimizing an activity of interest by fractionating a pool containing some degree of the desired activity to provide a set of smaller pools. Iterative deconvolution is achieved by repeating this method, which results in single members with a high level of activity.
Tagging
The most popular method for encoding solid phase libraries is to attach a chemical tag to the resin beads during the synthesis of the target molecule. Typically, a tag specific to the given phase is attached at each one of the reaction’s steps.
Electronic encoding
This method employs a radio frequency (RF) memory tag, which is a microelectronic device.
Advantages of combinatorial synthesis
- Combinatorial approaches can synthesize millions of compounds at the same time.
- A negative mixture result avoids the effort of synthesis, purification, and identification of each constituent.
- The isolation, purification, and identification of active molecules from a combinatorial library is relatively simple.
- Chemical pools are created by mixed combinatorial synthesis.
- In a random screening process, the probability of detecting a molecule is proportional to the number of molecules tested.
Disadvantages of combinatorial synthesis
- The size, solubility, and function group of a chemical all have a significant impact on its efficiency.
- Compounds generated are typically Achiral or Racemic.
References
- https://www.sciencedirect.com/topics/chemistry/combinatorial-chemistry#:~:text=Combinatorial%20chemistry%20uses%20chemical%20synthesis,for%20such%20large%2Dscale%20synthesis.
- https://www1.udel.edu/chem/C465/senior/fall00/DrugDiscovery/WhatisCombinatorialChemistry.htm
- https://www.slideshare.net/naresh2231989/combinatorial-chemistry-1
- https://slideplayer.com/slide/10583998/
- https://www.slideshare.net/PujaBhalerao2/combinatorial-chemistry-143066292
