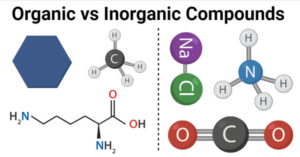
Organic vs Inorganic Compounds- Definition, 13 Key Differences, Examples
Organic Compounds Definition Organic compounds are chemical compounds composed of one or more carbon atoms bonded to other elements like hydrogen, oxygen, or nitrogen. Organic compounds consist of multiple carbon-hydrogen … Read more