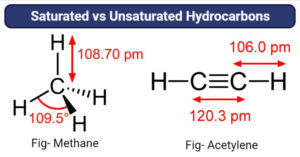
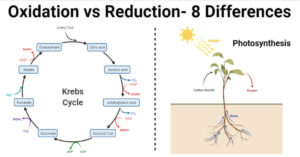
Differences between chemisorption and physisorption
Physical adsorption, also known as physisorption, is a reversible phenomenon involving weak Van der Waals forces between adsorbate and adsorbent, whereas chemisorption is an irreversible phenomenon involving strong chemical interaction … Read more