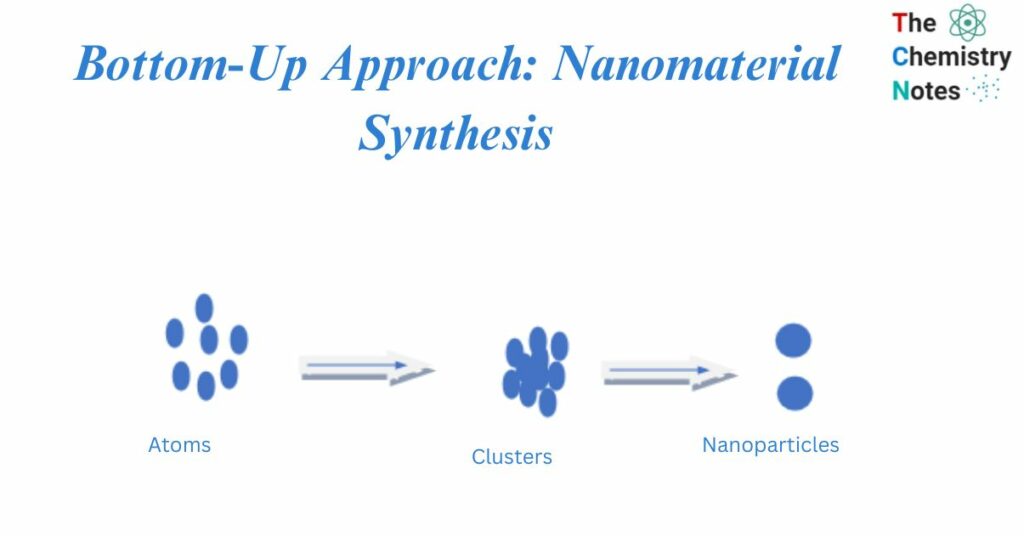
The Bottom-up approach utilizes and combines sub- or nanoscale items (atoms or molecules) to create nanostructures with new or different functions. This strategy, which enables more controlled system design, can be seen as the logical progression of supramolecular chemistry, which focuses on intermolecular links and examines the structures and functions of the entities created by the association of two or more chemical species The ‘bottom-up’ strategy, on the other hand, has the potential to produce less waste and hence be more cost-effective. Bottom-up approach refers to the construction of material from the ground up: atom by atom, molecule by molecule, or cluster by cluster.
The primary drawback of Bottom-up approach is the complex chemistry of supramolecular or multi-component structures, which frequently makes large-scale synthesis problematic.
The bottom-up approach, or self-assembly, techniques for nanofabrication use nanoscale chemical or physical forces to build basic units into bigger structures. The bottom-up approach is becoming an increasingly significant complement to top-down techniques in nanofabrication as component size reduces.
Methods of Bottom-up Approaches
Chemical vapor deposition method
During Chemical vapor deposition, a thin coating is formed on the substrate surface via a chemical process involving vapor-phase precursor. Chemical vapor deposition technologies are extremely important in the production of carbon-based nanomaterials. A precursor is regarded ideal for CVD if it has sufficient volatility, high chemical purity, strong evaporation stability, cheap cost, is non-hazardous, and has a long shelf life. Furthermore, its decomposition should not produce leftover contaminants.
Hydrothermal method
One of the most popular and frequently used processes for producing nanostructured materials is the hydrothermal process. The hydrothermal method produces nanostructured materials by performing a heterogeneous reaction in an aqueous medium at high pressure and temperature near the critical point in a sealed vessel.
Solvothermal method
The solvothermal approach works similarly to the hydrothermal method. One difference is that it takes place in a non-aqueous medium.
Sol-gel method
A colloidal solution of particles suspended in a liquid phase is referred to as a sol. A gel is a solid macromolecule that is submerged in a solvent. Sol-gel is the most popular bottom-up approach due to its simplicity and the ability to synthesize the majority of nanoparticles. It is a wet-chemical method that uses a chemical solution as a precursor to an integrated system of discrete particles. Metal oxides and chlorides are the most common sol-gel process precursors.
In solution, metal alkoxides or metal precursors are condensed, hydrolyzed, and thermally degraded. The result is a stable solution, often known as a sol. Hydrolysis or condensation increases the viscosity of the gel. Adjusting the precursor concentration, temperature, and pH levels can reveal the particle size.
Spinning
A spinning disc reactor is used to synthesize nanoparticles through spinning. It consists of a revolving disc contained within a chamber/reactor where physical factors such as temperature can be regulated. To remove oxygen from the reactor and avoid chemical reactions, it is usually filled with nitrogen or other inert gases. The liquid, i.e. precursor and water, is injected into the disc while it rotates at different speeds. The spinning causes the atoms or molecules to fuse, which causes them to precipitate, gather, and dry.
Co-precipitation
It is a wet chemical treatment that uses a solvent displacement approach. Solvents include ethanol, acetone, hexane, and non-solvent polymers. Synthetic and natural polymer phases are both possible. Fast diffusion of the polymer-solvent into the polymer’s non-solvent phase results from mixing the polymer solution. The production of nanoparticles is caused by interfacial tension between two phases.
Soft and Hard template method
Nanoporous materials are produced using soft and hard template techniques. The soft template approach is a straightforward traditional method for creating nanostructured materials. Soft templates, such as block copolymers, flexible organic molecules, and anionic, cationic, and non-ionic surfactants, are used to create nanoporous materials in the soft templating process. Hydrogen bonding, van der Waals forces, and electrostatic forces are the most prevalent interactions between the soft templates and the precursors. To create 3D-ordered mesoporous structures, soft templates of 3D specially structured liquid crystalline micelles are employed.
Nano-casting is another name for the hard template process. To achieve nanostructures for necessary applications, well-designed solid materials are utilized as templates, and the solid template pores are filled with precursor molecules. The hard template must be chosen carefully if well-ordered mesoporous materials are to be created. Such hard templates should have a mesoporous structure during the precursor conversion process and be easily detachable without affecting the resulting nanostructure. As hard templates, a variety of materials, including carbon black, silica, carbon nanotubes, particles, colloidal crystals, and wood shells, have been employed.
Pyrolysis
Pyrolysis is the most often utilized process in industry for large-scale nanoparticle manufacturing. It entails using a flame to burn a precursor. The precursor is either a liquid or a vapor that is introduced into the furnace under high pressure through a tiny opening and burned. The combustion or by-product gases are then air-categorized to recover the nanoparticles. Pyrolysis has the advantages of being a simple, efficient, cost-effective, and continuous process with a high yield.
Inert gas condensation
This approach produces a substantial amount of metal NPs. The inactive gas compression technique, which makes NPs by causing a metallic source to disappear in an inert gas, has been widely used.
Reverse micelle approach
The reverse micelle approach can also be used to create nanomaterials with specific shapes and sizes. An oil-in-water emulsion produces normal micelles with hydrophobic tails aiming at a core containing trapped oil droplets. In the case of a water-in-oil emulsion, however, reverse micelles form when the hydrophilic heads point towards a water-containing core. The reverse micelle core functions as a nanoreactor for the creation of nanoparticles. It serves as a reservoir for the development of nanomaterials. The size of these nanoreactors can be adjusted by adjusting the water-to-surfactant ratio, which in turn affects the size of the nanoparticles synthesized using this approach.
Green synthesis
The synthesis of different kinds of metal nanoparticles with bioactive agents such as plant materials, microorganisms, and various biowastes such as vegetable waste, fruit peel waste, eggshell, algae, and so on is referred to as “green” or “biological” nanoparticle synthesis. To avoid the development of undesired or toxic byproducts, it is vital to develop sustainable green synthesis processes. Green nanoparticle synthesis has various advantages, including being simple and inexpensive, creating NPs with high stability in a short period, and producing non-toxic byproducts.
Advantages of the Bottom-Up approach
- The bottom-up approach can produce ultra-fine nanoparticles, nanoshells, and nanotubes.
- Deposition parameters can be adjusted during synthesis.
- A narrow size distribution (1-20 nm) is achievable.
- The bottom-up approach is a less expensive procedure.
Disadvantages of the Bottom-Up approach
- Using the Bottom-up approach, large-scale production is challenging.
- Nanoparticles must be chemically purified.
References
- https://ccsuniversity.ac.in/bridge-library/pdf/L-3%20Synthesis%20of%20Nanostructured%20Materials%20Prof%20BPS.pdf
- http://www.lscollege.ac.in/sites/default/files/e-content/Bottom%20up%20Synthesis%20of%20Nanomaterials.pdf.
- https://www.mdpi.com/2304-6740/9/7/58
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10168541/
- https://journals.innovareacademics.in/index.php/ijcpr/article/view/41556/24630
