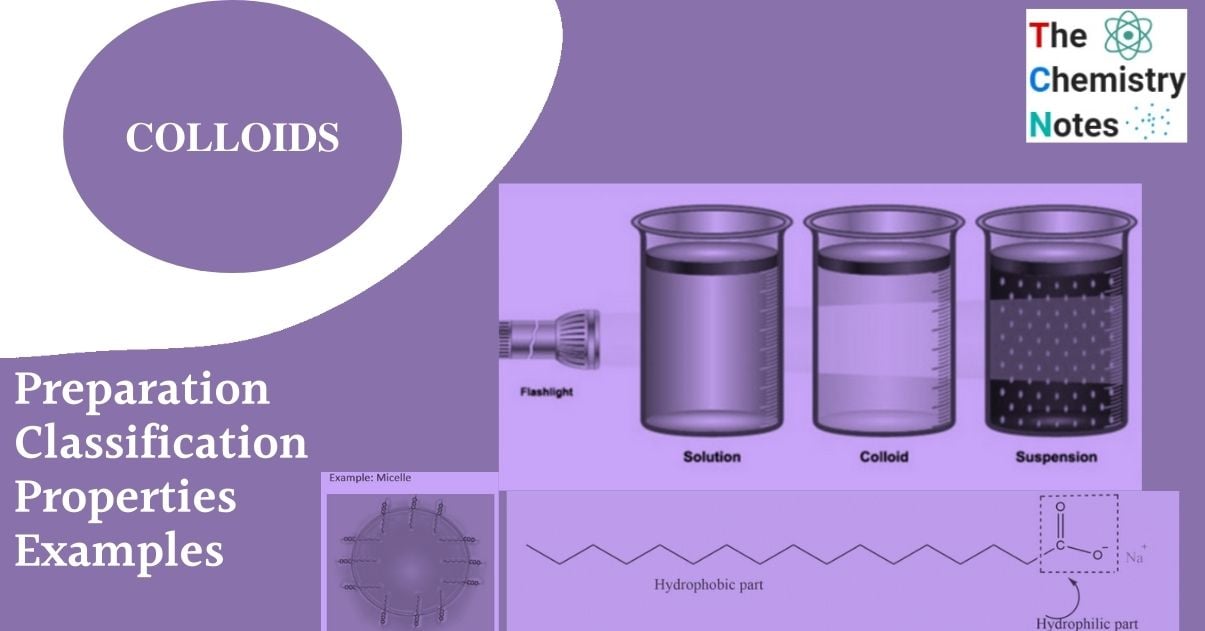
Colloids are substances with particle sizes between 1 and 1000 nm. Colloidal solutions or colloidal dispersions are a type of solution that exists between true solutions and suspensions. In another way, the diameter of the dispersed particles in a colloidal dispersion is greater than that of the solute particles in a true solution but less than that of a suspension. When the diameter of the particles of a substance dispersed in the solvent ranges from about 10 A to 2000 A, it is termed a colloidal solution or colloidal dispersion, or simply colloid.
A colloidal system consist of two phases.
- Dispersed phase: The substance is distributed as colloidal particles. E.g., dust particle (in air)
- Dispersed medium: The continuous phase in which the colloidal particles are dispersed. E.g., water (in milk)
A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) in another substance known as the dispersion medium.
Preparation of colloid
Chemical method
Double decomposition method
Hydrogen sulfide, on passing through a solution of arsenious oxide in distilled water gives a colloidal solution of arsenious chloride.
As2O3 + 3H2S → As2S3 (Sol) + 3H2O
Oxidation method
Hydrogen sulfide on passing through an aqueous solution of sulfur dioxide produces a colloidal solution of sulfur. Alternatively, hydrogen sulfide on passing through the solution of an oxidizing agent, like bromine water and nitric acid, and also gives a colloidal solution of sulfur.
SO2 + 2H2S → 2H2O + 3S (Sol)
H2S + [O] → H2O + S (Sol)
Reduction method
Reduction of the salt solution of these metal such as silver, gold as well as platinum produces their coolidal solution. For example,the reduction of gold trichloride gives the colloidal sol of gold.
2 AuCl3 + 3 HCHO + 3H2O → Reduction 2Au(sol) + 3HCOOH + 6HCl
By hydrolysis
On hydrolysis, ferric chloride gives the ferric hydroxide.
FeCl3 + 3H2O → Fe(OH)3 (sol) + 3HCl
Physical method
Exchange of solvent
It is the process of forming a colloidal solution of an element by adding its alcoholic solution to excess water. This colloidal formation can occur only if the element is more soluble in one solvent than in other.
From excessive cooling
This method involves freezing a water solution in organic solvents such as chloroform, ether, and others to form colloidal ice solutions.
Dispersion method
Mechanical dispersion
Solid and liquid dispersion mediums are poured into the colloidal mill to give colloidal sol.
Peptization
It is the process that involves the conversion of a precipitate into colloidal sol by shaking it with a dispersion medium in presence of a small amount of electrolyte. The electrolyte used for this purpose is called a peptizing agent.
Bredig’s Arc method (Electrodispersion)
In this method, an electric arc is formed between metal electrodes immersed in the dispersion medium. The intense heat generated, vapourises the metal, which then condenses to form colloidal particles. Sols of metals like gold silver and platinum can be prepared from this method.
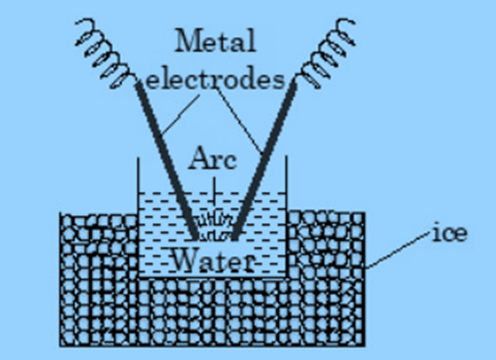
source : https://thechemistryguru.com/neet/surface-chemistry-colloidal-solution/
Classification of colloid
The colloid can be classified into different types based on the state of the dispersed phase and dispersion medium, the nature of the interaction between them, and the types of particles of the dispersed phase.
Based on the physical state of the dispersion medium and dispersed phase
The colloids can be classified into eight types based on whether the dispersion medium and dispersed phases are solid liquid and gas. These are as follows:
Solid sol
A colloid containing one solid dispersed in another continuous solid. E.g., gemstones, and colored gases.
Solid sol
A colloid in which gas is dispersed in a continuous solid medium. E.g., pumice stone. foam rubber
Sol
Sol is a colloidal system in which a solid is dispersed in a liquid. E.g., paints, cell fluids
Aerosol
It consists of solid as the dispersed phase and gas as the dispersion medium. E.g., smoke, dust.
Aerosol
Another type of colloidal system which consists of liquid as the dispersed phase and gas as the dispersion medium. E.g., fog, mist, cloud
Gel
It is the colloidal system in which the liquid is dispersed into a solid. E.g., cheese butter, jellies
Emulsion
It is a colloidal system in which both dispersed phase and dispersion medium are liquids. E.g., Milk, hair serum
Foam
It is a type of colloidal system having gas as the dispersed phase and liquid as the dispersion medium. E.g., froth whipped cream
Out of various colloids, the most common colloids are sol, gel, and emulsion.
Based on the nature of the interaction between the dispersion medium and dispersed phase
Colloidal sols are classified as lyophilic (solvent attracting) or lyophobic (solvent repelling) based on the nature of the interaction between the dispersed phase and the dispersion medium. When water is used as the dispersion medium, the terms hydrophilic and hydrophobic are used.
a. Lyophilic sols
Lyophilic sols are those in which the dispersed phase exhibits a definite affinity for the dispersion medium or solvent. If the dispersion phase and medium are separated, the sol can be easily regenerated by mixing them. So, they have also known as reversible sols. They are stable and cannot coagulate easily.
b. Lyophobic sols:
Lyophobic sols are those in which the dispersed phase has no attraction for the dispersion medium or solvent. These sols are not stable and can be easily precipitated (or coagulated) when small amounts of electrolytes are added, heated, or shaken. Furthermore, once precipitated, they do not return colloidal sol by simply adding the dispersion medium. As a result, these sols are also known as irreversible sols.
Based on the types of particles of the dispersed phase
Colloids are classified as multimolecular, macromolecular, and associated colloids according to the type of particle in the dispersed phase.
1. Multimolecular colloids
A large number of atoms or smaller molecules of a substance aggregate together during dissolution to form colloidal species (1–1000 nm). The species thus formed are called multimolecular colloids. Thousands or more of S8 molecules are present in the sulfur sol.
2. Macromolecular colloids
In a suitable solvent, the macromolecules form a solution in which the size of the macromolecules may be in the colloidal range. Such systems are known as macromolecular colloids. They are quite stable and resemble true solutions in many ways.
3. Associated colloids
The spontaneous aggregation or association of a large number of individual molecules in a given solvent forms Associated colloids. For example, micelle is a common type of associated colloidal.
Formation of micelle
Soap is the sodium or potassium salt of higher fatty acid, and can be represented as RCOO–Na+. When it dissolves in water it dissociates into RCOO- and Na+. The RCOO- ions, on the other hand, consist of two parts: a long hydrocarbon chain R (also known as a non-polar ‘tail’) that is hydrophobic (repels water) and a polar group COO- that is hydrophilic (water-loving).
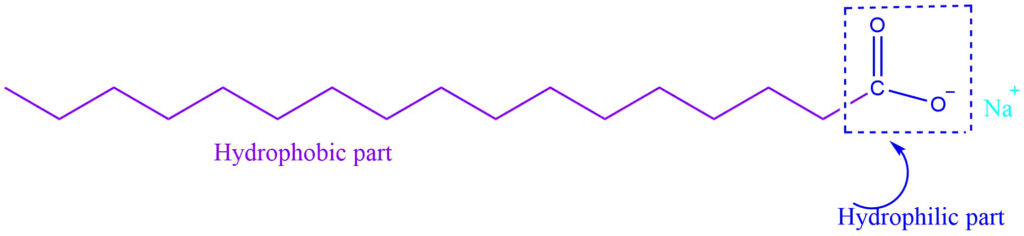
As a result, the RCOO- ions are present on the surface with their COO- groups in water, while the hydrocarbon chains R stay away from it and remain at the surface. At critical micelle concentration, the individual entities aggregate to form a spherical shape, with their hydrocarbon chains pointing towards the center of the sphere and the COO- part extending outward on the sphere’s surface. These aggregates are also known as a micelle.
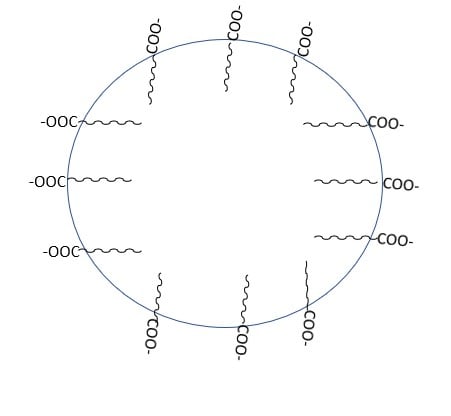
The cleansing action of soap and detergent
When water comes in contact with grease spots or dirt particles, the grease and dirt particles cannot be removed due to the interfacial tension created between the two phases. When detergent soap or detergent is added, their particles are adsorbed on the water surface which decreases the surface tension of the water. As a result, the interfacial tension is minimized. When interfacial tension is minimized two phases start to mix and hence gas spots or dirt particles can be easily removed by washing.
Alternatively, when soap and detergents are dissolved in water, they dissociate into RCOO- and Na+. At critical micelle concentration RCOO- a group of soaps starts to aggregate and form a globular structure called a micelle. In micelle, the long chain non-polar hydrocarbon tail faces inwards but the polar group faces outward. When such micelle comes in contact with the grease spot then the non-polar hydrocarbon tail of the micelle attacks the grease spot. So that emulsification of the grease spot occurs. Such emulsified grease spots and dirt particles can also be easily removed by washing.
Emulsions
These are the colloidal system in which both dispersed phase and dispersion medium are liquids. These are the liquid-liquid colloidal system. Generally, there are two types of emulsions, they are:
Oil in water type emulsion: The type of emulsion in which oil acts as the dispersed phase and water acts as a dispersion medium. E.g., Milk
Water and oil type of emulsion: The type of emulsion in which water acts as the dispersed phase and oil acts as a dispersion medium. E.g., Curd
Preparation of emulsion
Emulsions can be prepared by agitating a small portion of one liquid with the bulk of other or by passing the mixture through a colloidal mill known as a homogenizer. Emulsions prepared by shaking the mixture of two liquids are generally unstable due to interfacial tensions between the two layers. So certain amount of other substances or lyophilic sols is added to increase the stability.
Properties of emulsions
1. Demulsification
On heating, freezing, centrifuging, or by the addition of an appreciable amount of electrolytes, the emulsion breaks into the individual constituent liquid. This is known as demulsification.
2. Dilution
The emulsion can be diluted with any amount of dispersion medium. On the other hand, the dispersed liquid when mixed with it will form a separate layer. This property of emulsion is also used to detect the type of given emulsion.
Gels
Gels are a type of colloidal system in which liquid is dispersed in the interconnecting network of a solid. A gel is a jelly-like colloidal system. Gels can be prepared by different methods. They are as follows:
- Cooling
- Metathesis or double displacement
- By change of solvent
Types of gel
There are two different types of gels they are:
Elastic gel
Elatic gels are those whose completely dehydrated form can be regenerated by adding water. They change their shapes when force is applied to them. On releasing the force, elastic gels regain their original shape. E.g., Agar-Agar gel
Non-elastic gel
If a gel’s completely dehydrated form cannot be regenerated by adding water, it is said to be non-elastic. E.g., Silica gel
Properties of gel
Swelling
In addition of water to partially dehydrated elastic gel cause an increase in the volume of gel, due to the absorption of water. This is called swelling.
Syneresis
On long-standing some gels undergo shrinkage. This property is called syneresis.
Thixotropy
Some gels are semisolid at rest but become liquid sol when agitated. Thixotropy also refers to the reversible sol-gel transformation.
References
- Shaw D. J. (1992). Introduction to colloid and surface chemistry (4th ed.). Butterworth-Heinemann.
- https://byjus.com/chemistry/preparation-of-colloidal-solutions/
- https://www.toppr.com/guides/chemistry/surface-chemistry/preparation-of-colloids/#Dispersion_Techniques
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Solutions_and_Mixtures/Colloid
- https://ncert.nic.in/ncerts/l/lech105.pdf
- https://www.slideshare.net/makoye1954/2-colloidal-system
- https://www.chem.uni-potsdam.de/groups/kolloid/3_lehre/mops/v01_introduction.pdf
- https://www.vedantu.com/chemistry/classification-of-colloids
- https://www.geeksforgeeks.org/colloids/
- https://thechemistryguru.com/neet/surface-chemistry-colloidal-solution/
