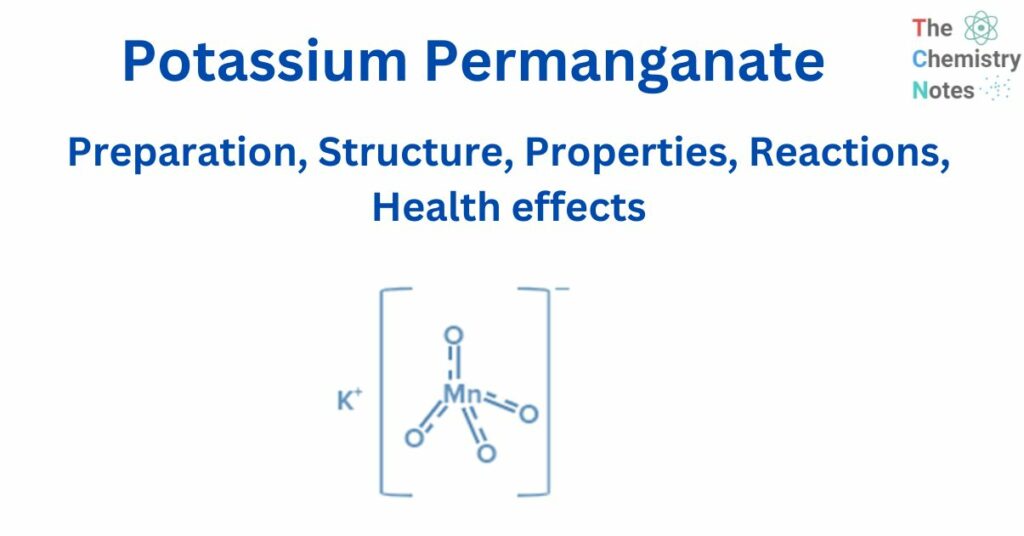
Potassium permanganate is a chemical compound with the chemical formula KMnO4. It is a manganic acid potassium salt. It is also known as Condy’s crystals or potash permanganate.
Johann Rudolf Glauber, a German-Dutch chemist, was the first to discover the KMnO4 in 1659. This water-soluble molecule is made up of two ions: permanganate ion and potassium ion. In its physical state, it is a dark purple odorless solid.
When potassium permanganate crystals are dissolved in water, a purple solution is generated. It is a potent oxidizing agent that does not produce harmful byproducts. It’s frequently made from other minerals like manganese oxide. Firstly, It was invented as a disinfectant. It has since been widely used to treat a wide range of skin diseases, including fungal infections.
Preparation of potassium permanganate
Potassium manganate is made by mixing a solution of potassium hydroxide and powdered manganese oxide with oxidizing agents such as potassium chlorate. After boiling and evaporating this mixture, the residue is heated in iron pans until it becomes pasty.
6 KOH + 3 MnO2 + 6 KClO3 → 3 K2MnO4 + 6 KCl + 3 H2O
- The resulting potassium manganate (green) is heated in a large amount of water, and a current of chlorine, CO2, and ozonized air is pushed through the liquid until it is transformed into permanganate. The generated MnO2 is continuously removed to prevent it from degrading the permanganate.
6 K2MnO4 + 3 Cl2 → 6 KMnO4 + 6 KCl3
- Reaction of potassium manganate with HCl to form potassium permanganate.
2K2MnO4 + 4HCl → 2KMnO4 + H2O + MnO2 + 4KCl
- Reaction of potassium manganate with H2SO4 to form potassium permanganate.
3 K2MnO4 + 2H2SO4 → 2KMnO4 + 2H2O + MnO2 + 2K2SO4
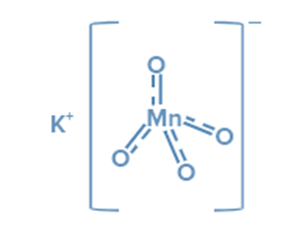
Structure of potassium permanganate
KMnO4 is an inorganic ionic compound composed of the ions K+ and MnO4–. In the permanganate anionMnO4- three double bonds and one single link connect the manganese atom to four oxygen atoms. Manganese has an oxidation state of + 7 in this salt. MnO4- ion hybridisation yields d3s (orbitals used are 3dxy, 3dyz, 3dzx, and 4s).
Tetrahedral geometry results from d3s hybridization. As a result, the Tetrahedral geometry of MnO4- can be expressed as
Physical properties of potassium permanganate
- It is a crystalline solid that ranges in color from purple to magenta and has no odor.
- It is soluble in water, acetone, acetic acid, methanol, and pyridine.
- It is soluble in ethanol as well as organic solvents.
- It occurs as monoclinic prisms that are almost opaque and have a blue metallic luster.
- It has no odor and a sweetish astringent taste in its aqueous solution. It is water-soluble and becomes more soluble in boiling water.
- It has a density of 2.7g/ml and its molar mass is 158.034g/mol.
- It has a high melting point of 2400 C.
- Potassium permanganate has a boiling point of 100°C.
Chemical properties of potassium permanganate
- Potassium permanganate is a powerful oxidizing agent that can be utilized in a wide range of chemical processes.
- During a redox reaction, the dark purple solution turns colorless and eventually brown, demonstrating potassium permanganate’s oxidizing power.
- This reaction might take place in an acidic or basic media.
- It is not combustible but aids in the combustion of other chemicals.
- It is a highly stable chemical under normal conditions, but upon heating it decomposes to generate MnO2 and liberates oxygen.
- It explodes when it comes into contact with sulfuric acid.
- It instantly interacts with glycerol and simple alcohols, producing flame and smoke.
Reactions of potassium permanganate
- Decomposition reaction
When heated, potassium permanganate undergoes thermal decomposition.
2 KMnO4 → K2MnO4 + MnO2 + O2
- Reaction with acid
When permanganate combines with strong hydrochloric acid, chlorine is formed. When potassium permanganate is reduced to an alkaline solution, it transforms into green K2MnO4.
2 KMnO4 + 16 HCl → 2 KCl + 2 MnCl2 + 5 H2O + 8Cl2
- Reaction with alkali
When potassium permanganate is heated with alkalies, it transforms into manganate and emits oxygen gas.
4 KMnO4 + 4 KOH → 4 K2MnO4 + 2 H2O + O2
- Oxidising properties
In acidic, neutral, and alkaline conditions, KMnO4 is a potent oxidizing agent. The following equations represent oxidation in certain media:
In an acidic medium:
2KMnO4 + H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O] MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
In a neutral or alkaline medium:
2KMnO4 + H2O → 2KOH + 2MnO2 + 3[O] MnO4– + 2H2O + 3e– → MnO2 + 4OH–
- Other reactions
With sulphuric acid
2 KMnO4+ H2SO4 → Mn2O7 + K2SO₄ + H2O
With hydrogen peroxide
2 KMnO4 + 3 H2O2 → 2 KOH + 2 MnO2 + 3 O2+ 2 H2O
With alkene
In a weak alkaline or neutral media, it can also oxidize carbon atoms with double bonds.
СH2 = СН2 + 2 KMnO4+ 2 H2O → CH2OH–CH2OH + 2 MnO2 + 2 KOH (with cooling).
Uses of potassium permanganate
- It is useful in leather tanning and fabric printing.
- It’s a bleaching agent, an antiseptic, and a pesticide.
- Acts as an oxidizing agent and aids in the synthesis of a variety of essential chemicals. Potassium permanganate has disinfectant properties. It can be used as a disinfectant to treat skin infections such as dermatitis, canker sores, ulcers, eczema, and fungal infections due to its oxidizing properties. When administered to minor wounds, it affects the microorganism’s outer cell membrane, oxidizing it and destroying its structure.
- It is used in water treatment plants to eliminate pollutants and remove unpleasant odors from water. It oxidizes iron, hydrogen sulfide, and manganese into solid particles, which are subsequently filtered out. Because the treatment does not produce any hazardous substances, it is quite safe to use.
- Because of its bright color and oxidizing properties, potassium permanganate is employed as a reagent in chemical labs to quantify the quantity of a substance that can be oxidized in a sample. This value is known as the permanganate value.
- It is useful for removing snails from plants before transferring them to an aquarium.
- It is a vital component of survival kits, along with glycerine/glucose tablets, and can be used to start fires. A fire can be started by rubbing glucose tablets with permanganate powder. It can also be used to clean wounds and water.
- It can be used as an antidote in cases of phosphorus poisoning.
- It’s frequently used to treat parasitic diseases in fish.
Health effects of Potassium permanganate
- When inhaled, it can irritate the nose and throat. Even the lungs can be damaged, resulting in coughing, shortness of breath, and pulmonary edema (fluid buildup in the lungs).
- KMnO
4may also affect the liver and kidneys. - Long-term exposure may have an impact on fertility.
- Potassium permanganate, in concentrated form, is irritating to the eyes and skin. It can react with a wide range of reducing agents and organic materials, however, it is flammable.
- The antibacterial effect of KMnO4 is based on the oxidation of bacteria or tissue proteins by this molecule. It stains the skin and tissues. Because it operates on all organic matter through a destructive oxidation process, its application is limited to external uses exclusively.
References
- Lee, J.D. (1996) Concise Inorganic Chemistry. 5th Edition, Chapman and Hall Ltd., London, 619-621.
- https://byjus.com/chemistry/kmno4/
- https://www.vedantu.com/chemistry/potassium-permanganate
- https://testbook.com/chemistry/kmno4
- https://unacademy.com/content/neet-ug/study-material/chemistry/preparation-of-potassium-permanganate-kmno4/
