Copper sulphate is an inorganic salt generated by the reaction of sulphuric acid and copper. This inorganic substance is a crystalline, odorless powder that absorbs water quite well. Anhydrous copper (II) sulfate is white and only turns blue when it comes into contact with water molecules. Copper sulphate pentahydrate is the most prevalent form, with the chemical formula CuSO4.5H2O. This type is distinguished by its intense blue color. it is commonly known as blue vitriol, Roman vitriol, copper vitriol, and bluestone.
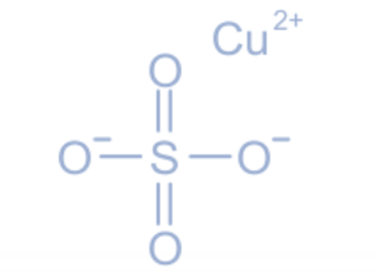
Blue vitriol is a copper salt with numerous industrial uses, including pesticide, fungicide, feed, and soil additives in agriculture, floatation reagent in zinc and lead recovery in mining, the blue and green pigment in dyes, print toner in photography, the production of other copper compounds, and leather tanning. It is found in the majority of multivitamins and mineral supplements. In the compound copper sulphate, an ionic link exists between the copper cation (Cu2+) and the sulphate anion (SO42-).
Preparation of copper sulphate
Hot, concentrated sulfuric acid is reacted with metallic copper at high temperatures to produce copper sulphate. Another way to make copper sulfate solution is to dissolve copper oxide in dilute sulphuric acid.
The following stages are involved in the manufacturing of copper sulphate pentahydrate:
i. Heap leaching, in which copper is extracted from oxidised ores using a sulfuric acid and water mixture.
ii. Solvent extraction, in which copper is extracted from the leaching solution by mixing with a product known as organic.
iii. Crystallisation, in which the copper-loaded organic is discharged using a concentrated acid solution.
iv. Re-crystallization, in which copper sulphate is dissolved in fresh water at 80 – 90 °C and subsequently crystallised by cooling to 25 – 30 °C to eliminate impurities.
Structure of copper sulphate
The crystal structure of anhydrous copper sulphate is orthorhombic, whereas the crystal structure of pentahydrate copper sulphate is triclinic. If one looks closely, copper sulphate appears to have a diamond form. Since copper and sulphate ions are bonded by the exchange of electrons, copper sulphate has an ionic bond. In copper sulphate, the oxidation state of copper is +2.
Properties of copper sulphate
Physical properties
- The molar mass of copper sulfate is 159.609 grams per mole, whereas the molar mass of copper sulfate penhydrate is 249.685 grams per mole.
- Copper sulfate is mostly found as a powdered substance, of which the anhydrous form, i.e. the form devoid of water molecules, is silvery white in color while copper sulfate pentahydrate is blue.
- One characteristic that both anhydrous and hydrated copper sulfate have in common is that they decompose on heating and do not have exact boiling points.
- Anhydrous copper sulphate has an orthorhombic crystal structure, whereas hydrous copper sulphate has a triclinic crystal structure.
- Blue vitrol is hygroscopic in nature and is non biodegradable.
Chemical properties
- Copper ions in copper sulphate often combine with hydrochloric acid chloride ions to form tetrachlorocuprate. This is typically accomplished in two processes, the first of which includes the dissociation of hydrochloric acid into hydrogen and chloride ions. After that, these chloride ions react with copper ions to form tetrachlorocuprate, where tetra chloro indicates four chlorine atoms.
4 HCl ⟶ 4 H+ + 4 Cl–
Cu2+ + 4 Cl− ⟶ (CuCl4)2−
- When copper sulphate pentahydrate is heated, it becomes anhydrous copper sulphate and releases five water molecules.
CuSO4.5H2O + Heat ⟶ CuSO4 + 5 H2O
- On additional heating, copper sulphate decomposes to create cupric oxide and sulphur trioxide.
CuSO4 + Heat ⟶ CuO + SO3
- Copper sulphate undergoes a displacement process in which the more reactive metal ion displaces the less reactive metal ion. E.g.,
Aqueous copper sulphate solution interacts with zinc to form zinc sulphate.
CuSO4 + Zn ⟶ Cu + ZnSO4
It depicts a single metal replacement process with iron sulphate.
CuSO4 + Fe⟶ Cu + FeSO4
- Copper sulphate produces an intense blue colour when combined with NH4OH resulting in a complex molecule.
CuSO4 + 4 NH4OH⟶ [Cu (NH3) 4]SO4 + 4 H2O
- When KI is added to a CuSO4 solution, it produces a white precipitate of cuprous iodide.
CuSO4 + 2 KI⟶ Cu I2 + K2SO4
(unstable)
2 CuI2 ⟶ Cu2I2 + I2
(white ppt)
- With KCN, a yellow precipitate of cupric cyanide is generated, which decomposes to produce cyanogen gas.
CuSO4 + 2 KCN ⟶ Cu(CN) 2 + K2SO4
2 Cu(CN) 2 ⟶Cu2 (CN) 2 + (CN) 2
cyanogen
- A pale blue precipitate of copper hydroxide is generated when alkalies are reacted with copper sulphate.
CuSO4 + 2 NaOH⟶ Cu(OH) 2 + Na2SO4
Pale blue precipitate
- When combined with H2S, it produces a dark precipitate of copper sulfide.
CuSO4 + H2S ⟶ CuS + H2SO4
(Black ppt)
Uses of copper sulphate
- Because copper sulphate dissolves easily in liquid, it is an effective cleanser for pools and reservoirs.
- It is used as a molluscicide in tropical areas.
- The most significant health benefit of copper sulphate is that it is used to reduce the growth of bacteria and fungi on fruits, vegetables, and other crops.
- In basic assays for the presence of water molecules in alcohol, copper sulphate pentahydrate is utilized.
- It is used to dry and remove mould from various surfaces.
- It is also used to examine blood samples for disorders such as anaemia.
- CuSO4 is combined with KMnO4 (potassium permanganate) to produce an oxidant.
- It is also used as a dye fixative in the vegetable dyeing process.
- It functions as an activator in the froth flotation concentration of lead, gold, and cobalt ores.
- It is used as an electrolyte in electrotype manufacturing and as an etching agent in print engraving. It serves as an electrolyte in the refinement of copper and the copper coating of steel wire prior to wire drawing.
- It is also used in anti-fouling paints and is crucial in glass coloring.
- The chemical is used in the synthetic fibre industry to produce raw materials.
- Copper sulphate solutions in water can be utilized as resistive element liquid resistors.
- It can also be used as a decorative material because it can bring color to cement, ceramics, and other metals.
- Copper sulfate is also added to bookbinding glues to keep insects away from the printed paper.
The health hazards of copper sulphate
- Copper sulphate may induce a burning or stinging sensation if absorbed through the skin or eyes. If introduced to the eyes, this could cause itching, eczema, conjunctivitis, inflammation, fluid buildup, or cornea irritation.
- It is well known that the reaction of copper sulphate with chlorates results in hazardous flames.
- Diarrhoea, nausea, vomiting, and profuse sweating can all result from copper sulphate intoxication.
References
- https://byjus.com/chemistry/cuso4/
- https://www.vedantu.com/chemistry/copper-sulphate-cuso4
- https://www.verywellhealth.com/copper-sulfate-benefits-4684436
- https://go.drugbank.com/drugs/DB06778
- https://testbook.com/chemistry-formulas/copper-sulphate-formula
