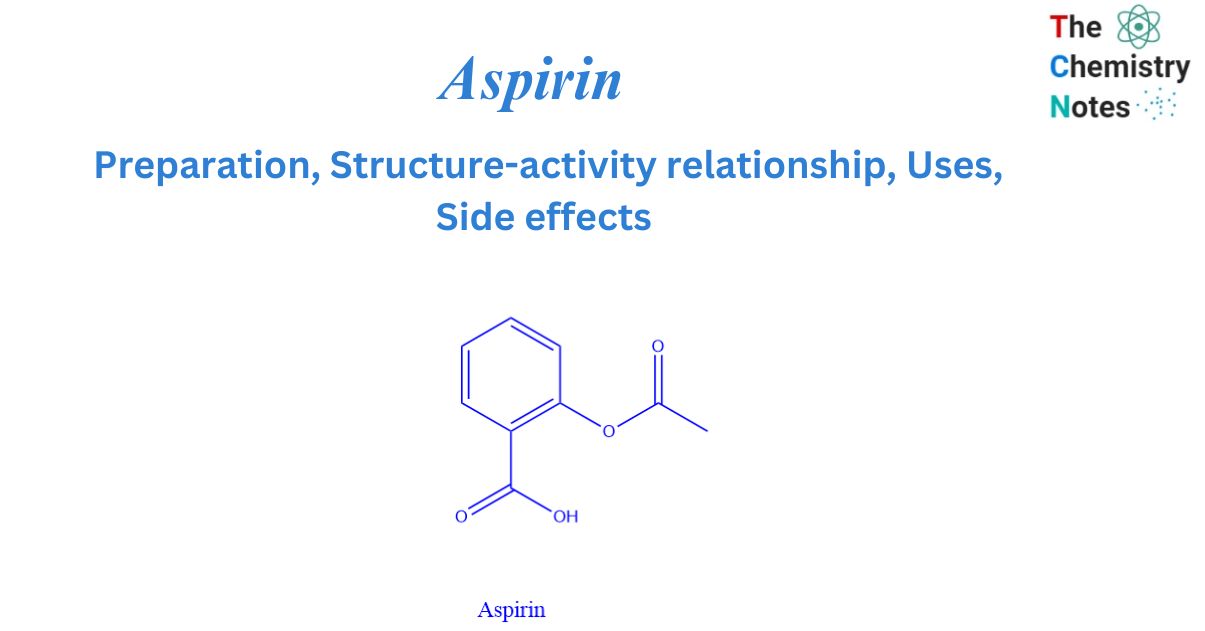
Aspirin is a nonsteroidal anti-inflammatory medication (NSAID). It is created chemically by acetylation with acetic anhydride from salicylic acid. Aspirin has a molecular weight of 180.16g/mol. It is colorless to white crystals or crystalline powder with no odor.
It is a drug that is widely used throughout the world. Acetylsalicylic Acid (ASA) is the chemical name for aspirin. It is still one of the oldest and most widely used forms of the medicine, which is commonly used as an anti-inflammatory and antipyretic prescription. It is a drug that is primarily used to treat pain, fever, and inflammation. Certain inflammatory disorders, such as Kawasaki illness, pericarditis, and rheumatic fever, are treated with aspirin.
Aspirin, also known as acetylsalicylic acid, is a well-known anti-inflammatory medication that suppresses prostaglandin formation by inhibiting the action of the enzyme cyclooxygenase. It decomposes quickly in solutions of ammonium acetate, citrates, carbonates, or alkali metal hydroxides.
It is a non-selective NSAID because it inhibits both cyclooxygenase (COX) enzymes involved in the conversion of arachidonic acid to prostaglandins and thromboxane irreversibly. As It is a non-selective COX-1 and COX-2 inhibitor, in addition to its analgesic, anti-inflammatory, anti-platelet, and antipyretic properties, it can cause peptic ulcers and gastrointestinal hemorrhage. It is thought to influence several cancer signaling pathways and may trigger or upregulate cancer suppressor genes3.
Structure Of Aspirin
Salicylic acid has two functional groups, one carboxylic acid (-COOH) and the other ester (-COOR). In an aqueous solution, the carboxylic acid group, as the name implies, has the ability to produce H3O+. Aspirin is planar in terms of molecular geometry. This causes a hybridization between the phenyl ring and carboxylic groups.
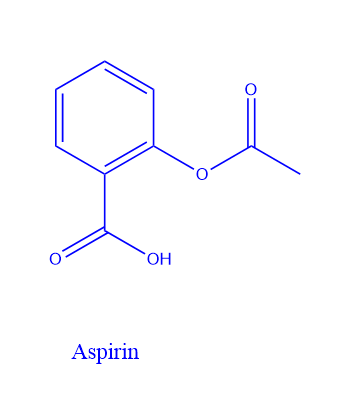
Occurrence of Aspirin
Acetylsalicylic Acid, like other chemicals, does not occur naturally in nature. It is not found in nature, and its history dates back to 1853. In that year, a French chemist named Charles Frédéric Gerhardt synthesized it for the first time.
Preparation of Aspirin
It can be prepared by esterification of Salicylic acid by acetic anhydride in the presence of sulphuric acid as a catalyst. As a result, the ester group replaces the hydroxyl group found in salicylic acid.
Structure-activity Relationship of Aspirin
- Carboxyl group substitution may impact efficacy and toxicity.
- By decreasing the acidity of the —COOH, the analgesic activity of salicylic acid is preserved, but the anti-inflammatory qualities are reduced.
- Attaching the phenolic hydroxyl group, meta or para to the carboxyl group decreases its activity.
- The addition of halogen atoms to the aromatic ring increases potency and toxicity.
- The substitution of aromatic rings at the 5 position of salicylic acid improves its anti-inflammatory action.
Uses of Aspirin
- Cyclooxygenase is inhibited by acetylsalicylic acid.
- It is employed in the prevention of both venous and arterial thrombosis.
- It is employed in the treatment of many forms of headaches.
- In veterinary medicine, It is used to manage pain in animals such as dogs and horses.
- It is a blood thinner that reduces the risk of cardiovascular disease in adults by preventing blood clots.
- It protects a person’s coronary artery against disease.
Side effect of Aspirin
- It is also used to prevent future heart attacks, ischemic strokes, and blood clots in those who are at high risk.
- It is also used to treat rheumatoid arthritis and Kawasaki disease.
- Convulsions, hallucinations, and fast breathing.
- Severe nausea, vomiting, or abdominal pain.
- When coughing, blood spills.
- Idiosyncrasy and hypersensitivity
- It can cause Reye’s syndrome in children, which causes liver and neurological system damage that can be life-threatening.
References
- https://byjus.com/chemistry/acetylsalicylic-acid/
- https://www.toppr.com/guides/chemistry-formulas/aspirin-formula/
- https://www2.chem21labs.com/labfiles/uofc_glaspirin_lab.pdf
- https://collegedunia.com/exams/acetylsalicylic-acid-structure-properties-and-uses-chemistry-articleid-2749
