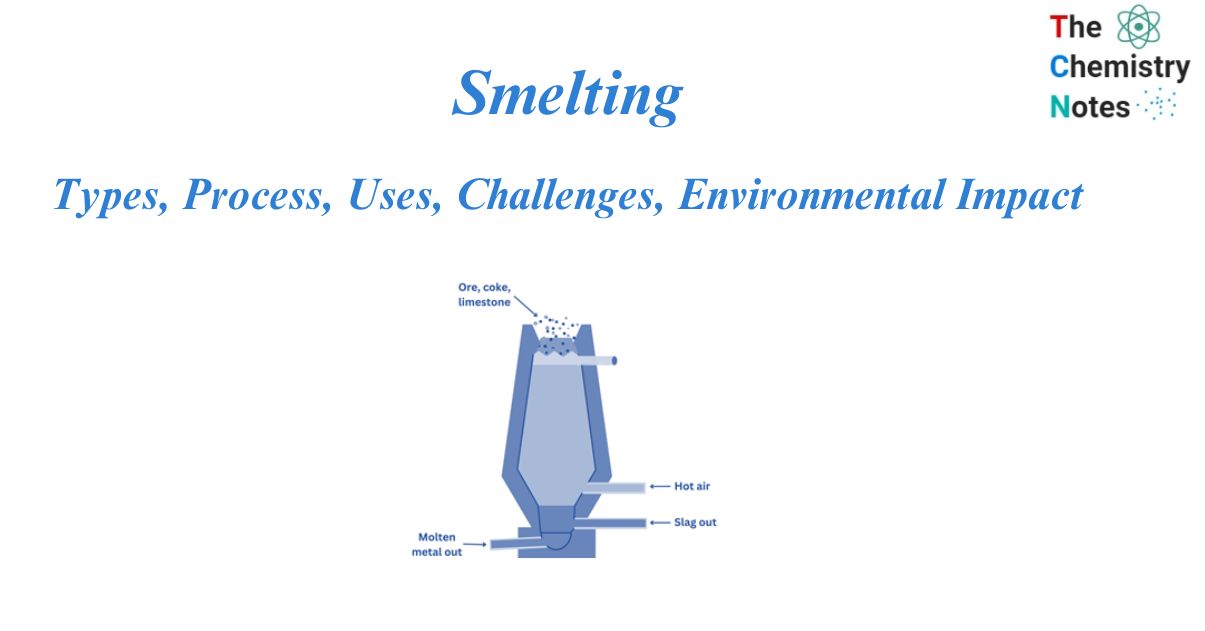
Smelting is the method of extracting a metal, either as an element or as a simple compound, from its ore by heating it above its melting point, usually in the presence of oxidizing agents such as air or reducing agents such as coke. The method was developed in the Middle East around 5000 BCE, when the first metal, copper, was smelted. Metal discoveries like copper and bronze had a huge impact on human history.
What is smelting?
It refers to the procedure of subjecting roasted, calcined ore to high temperatures in the presence of a reducing agent, typically charcoal, and an appropriate flux. This process is carried out above the melting point of the ore.
It’s a type of extractive metallurgy. It is used to extract various metals from their ores, including copper, aluminum, iron, silver, lead, and many other base metals. It employs heat and chemical-reducing agents to decompose the ores, which eliminates additional elements in the form of gases or slag, leaving behind the metal base.
Types of smelting process
Reduction smelting
In the reduction smelting process, oxidic metal sources are reduced with carbon in the presence of a flux.
Matte Smelting
The matte smelting technique combines sulfidic metal sources with a flux without the use of a reducing agent. In comparison to metal oxide smelting, matte smelting can be done at a lower melting point.
Flash Smelting
The flash smelting process combines the flash roasting and smelting operations into one. At high temperatures, the sulfide concentrate particles react with oxygen. The oxidation process creates enough heat to allow the smelting process to take place simultaneously.
Processes of smelting
It often includes the following steps:
- The process of reducing metal using carbon
- Impurity Removal through Slag Formation
The heat required for the process is produced within the furnace by the combustion of carbon (C) in the presence of a high-temperature stream of air. This technique makes use of either blast furnaces or reverberatory furnaces.
Historically, reducing agents for this process were carbon-based fossil fuels such as coke or charcoal. Because the bonds in carbon dioxide have low potential energy at high temperatures, oxygen in the ore binds to carbon.
Carbon is used as a chemical reactant to remove oxygen from the ore, which results in the purified metal element as a product.
The process of reducing metal using carbon
Metal oxides formed through calcination or roasting procedures are combined with carbon to undergo reduction and conversion into metal using a dry extraction approach.
When carbon reacts with oxygen in the air, carbon monoxide is formed. After numerous repeated interactions of carbon monoxide with the ore’s oxygen, the whole oxygen would be eliminated, leaving only the raw base metal.
CuO + C Δ → Cu + CO
SnO2 + 2 C Δ → Sn + 2 CO
Fe2O3 + 3 C Δ → 2 Fe + 3 CO
Carbon monoxide (CO) can be generated through incomplete combustion of carbon, leading to the reduction of the oxide to its elemental form.
Fe2O3 + 3 CO Δ → 2 Fe + 3 CO2
Advantages of carbon as a reducing agent
(i) It is the most cost-effective of the reducing agents offered.
(ii) The substance can be easily pulverized and mixed.
(iii) Carbon monoxide (CO) can be produced as a gaseous reducing agent and then used to reduce the metal oxides found in the furnace’s top part.
(iv) Carbon combustion is recognized for releasing a substantial amount of heat energy, making it an appropriate source for providing the necessary thermal energy in this process.
Impurity Removal through Slag Formation
In numerous instances, alongside carbon, supplementary substances known as flux are introduced to facilitate the elimination of non-melting impurities (referred to as matrix) by forming a molten mass called slag.
Due to its immiscible nature and lower density compared to molten metal, the slag flows to the surface and can be readily removed through blowing or runoff.
As an example, the removal of silica can be achieved through the addition of lime as a flux.
CaO + SiO2 → CaSiO3
Flux Impurity Slag
Flux
Flux is a material added to ores before heating to enable a chemical reaction with the impurities, also known as gangue, found within the ores. This reaction produces slag, which is capable of melting at lower temperatures.
Metal workers in smelting utilize flux for a variety of applications. Catalyzing a reaction and chemically bonding the impurities and reaction product with the metal is the most prevalent.
There are three distinct types of fluxes:
(i) Acidic fluxes such as silica (SiO2), borax (Na2B4O7), P2O5, and others, are commonly used in various applications.
(ii) Basic fluxes include limestone (CaCO3), magnesite (MgCO3), haematite (Fe2O3), and others.
(iii) Neutral fluxes such as calcium fluoride (CaF2), are commonly employed to preserve the fluidity of molten substances.
Challenges of a smelting process
This process can be a difficult technique. First off, because searching for ore is challenging, there is no assurance that you will smelt the desired metal. The entire process of gathering and smelting iron ore could result in the production of impurities and slag.
Second, forging newly extracted metal into any shape is a time-consuming process. To shape a piece of metal, you may need to hammer it for hours, reheat it, and then pound it again.
In this process, the choice of slag composition is critical to achieving the best combination of basicity and fluidity: maximal impurity elimination.
Applications of smelting process
- Many metals, including silver, iron, copper, and base metals, are extracted from their ores using it.
- Metal smelting is used in a variety of industries in their day-to-day operations and manufacture. Steel, a crucial manufacturing product for a wide range of industries, is mostly produced through smelting.
Environmental Impact of a smelting process
- The smelting business has a negative impact on the environment because it generates a lot of slag and wastewater, which is released into the environment together with hazardous metals such as copper, silver, iron, cobalt, and selenium.
- Water is utilized extensively in this process operations for cooling, cleaning, and other purposes. As a result, effluent containing metals, acids, and other pollutants may be discharged, harming aquatic life and creating health risks if not treated correctly.
- During this process, several gases and particulate matter are released into the environment, including sulfur dioxide (SO2), nitrogen oxides (NOx), carbon monoxide (CO), carbon dioxide (CO2), and metal dust. Sulfur dioxide, for example, can generate acid rain, which affects ecosystems, soil, and water bodies. Smog is caused by nitrogen oxides, which are damaging to human respiratory systems.
- Smelting metals from ores deplete nonrenewable resources like metal ores and fossil fuels, which can have long-term environmental consequences.
- Workers in the smelting sectors have been documented to develop respiratory illnesses, which impair their capacity to conduct the physical activity required by their jobs in the smelting business.
References
- https://www.britannica.com/technology/smelting.
- https://www.vedantu.com/chemistry/smelting
- https://blog.thepipingmart.com/metals/copper-smelting-an-overview/
- https://slideplayer.com/slide/6817540/
- https://www.slideshare.net/slideshare1869/smelting-furnaces
