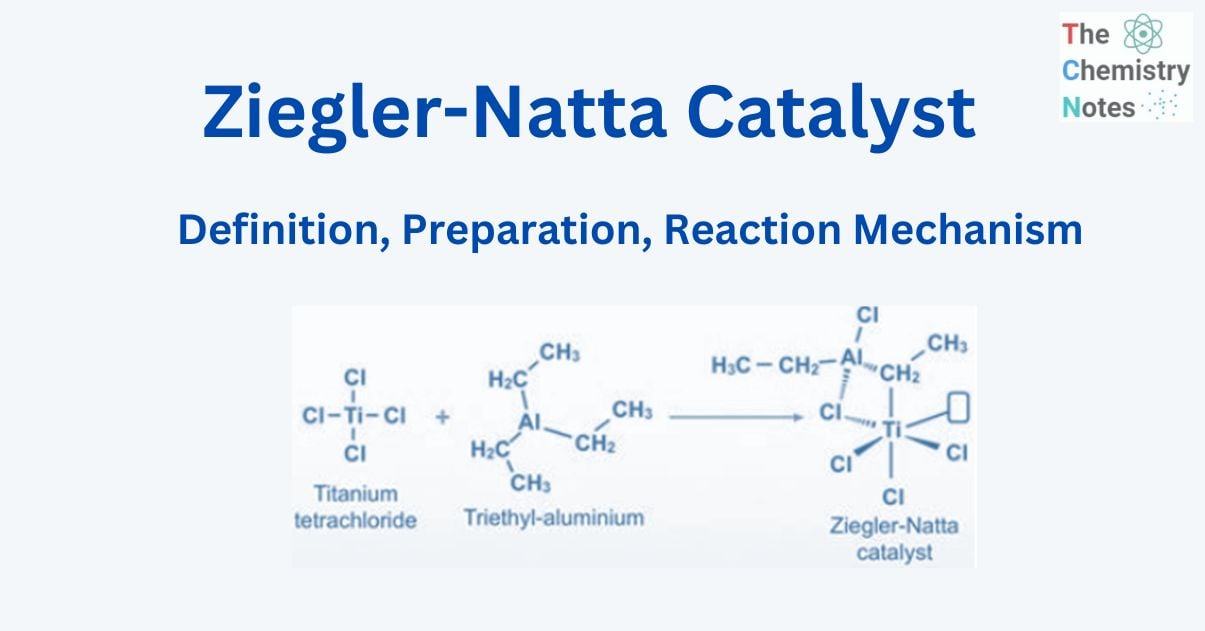
The Ziegler-Natta catalyst is named after Karl Ziegler and Giulio Natta and is used to polymerize poly 1-alkene. It is an important class of chemical compounds notable for their capacity to polymerize olefins (hydrocarbons with a double carbon-carbon bond) to polymers with large molecular weights and highly ordered (stereoregular) structures.
This catalyst is employed because it is extremely stereoselective and creates a wide range of useful polymers. A Ziegler-Natta catalyst is made from titanium tetrachloride TiCl4 and diethyl aluminum chloride [(C2H5)2AlCl].
Based on his discoveries about the mechanics of the polymerization reaction, Giulio Natta, an Italian scientist, expanded the approach to other olefins and produced other versions of the Ziegler catalyst. Many Ziegler-Natta catalysts are combinations of transition metal halides, including titanium, chromium, vanadium, and zirconium, with organic nontransition metal derivatives, particularly alkyl aluminum compounds.
Interesting Science Videos
Preparation of Ziegler-Natta catalyst
Transition metal halides from groups 4 to 8 of the contemporary periodic table regularly combine with organometallic compounds from groups 1 to 3 to generate Ziegler-Natta catalysts, with titanium tetrachloride and trimethylaluminum being two common examples.
Ziegler Natta catalysts feature intricate structures that include coordination chemicals. They frequently produce active sites that aid in the polymerization process. The spatial organization and reactivity of the catalyst are influenced by the ligands and metals used.
Types of Ziegler-Natta catalyst
Ziegler-Natta catalysts are the most widely used in the global polymerization industry. The Ziegler-Natta catalyst has been classified into two major classes based on solubility:
Heterogeneous catalysts
These are industry-leading catalysts based on titanium compounds (and sometimes vanadium compounds) and utilized for polymerization reactions, typically used together with organo-aluminum compounds such as tri-ethylaluminium as co-catalysts.
Titanium tetrachloride is supported on magnesium chloride by tri-ethylaluminium (AlEt3) or AlEt2Cl as co-catalysts in heterogeneous Ziegler-Natta catalysts. Lewis bases such as ethyl benzoate, silanes, or other donors are used to improve the stereo control of the propylene polymerization process. Because heterogeneous catalysts are complicated systems with multiple active sites, the polymer structure is very slightly affected.
Homogeneous catalysts
The second broad category of catalysts is based on complexes of Ti, Zr, or Hf. They are typically employed in combination with a variety of metallocene/methylaluminoxane organo-aluminum co-catalysts. They traditionally contain metallocenes, although they also contain multi-dentate oxygen- and nitrogen-based ligands.
Polymerization reactions using Ziegler-Natta catalysts
This chemical has a crystal structure because titanium atoms are coupled with six chlorine atoms. It receives one ethyl group from AlEt3 when it interacts with it. Aluminium bonds with a chlorine atom. In titanium compounds, one chlorine atom is eliminated. As a result, the catalyst now has an empty orbital on its surface. The coordination of AlEt3 with titanium now activates the catalyst.
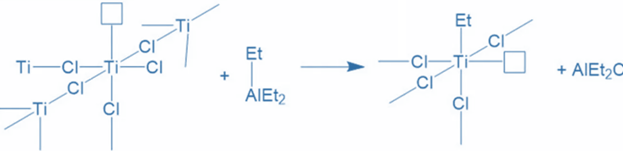
Initiation
The pair of electrons from the carbon-carbon pi-bond transfer to produce the Ti-carbon bond, while the pair of electrons from the Ti-AIEt3′ ethyl group shift to form a bond between the ethyl group and the methyl-substituted carbon of propylene. Ti now has an empty orbital due to electron shuffling and requires electrons to fill it.
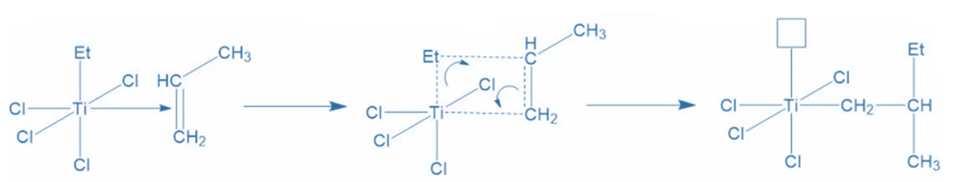
Propagation
The reaction’s propagation is dependent on the availability of free propylene molecules. As more propylene molecules enter the system, the process is repeated, resulting in linear polypropylene.
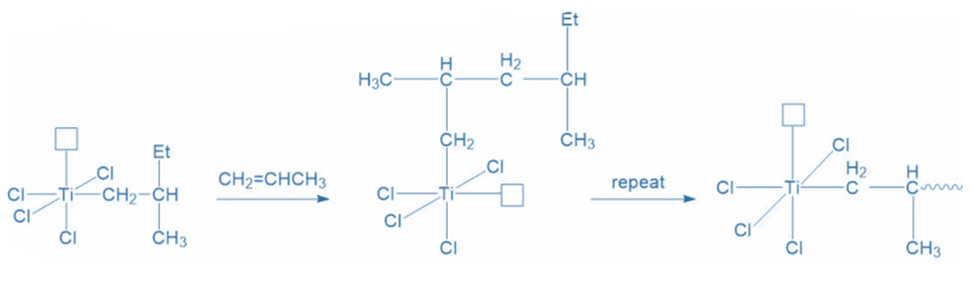
Termination
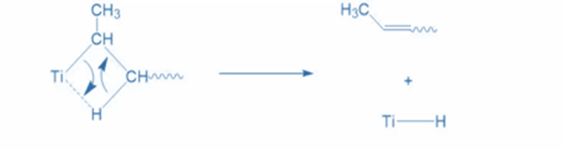
Termination is the ultimate stage in the chain-growth polymerization process, resulting in desired products. Several termination strategies have been developed with the help of co-catalyst AIEt3.
Importance of Ziegler-Natta catalyst
- It is highly efficient and stable.
- High stereoregularity (99% tacticity)
- High polymer product concentration.
- Simple regeneration.
- Lower production costs.
- Regulates polymer product growth and production.
Applications of Ziegler-Natta catalyst
It is used for various applications, which include:
- High-density polyethylene production.
- Manufacturing of linear Low-density polyethylene.
- The manufacture of thermoplastic polyolefins.
- Crystalline polypropylene production.
- Carbon nanotube composites.
Limitations of Ziegler-Natta catalyst
Because it does not work for all monomers, the Ziegler-Natta polymerization has some limitations. Poly(vinyl chloride) and acrylates are two products that cannot be produced via Ziegler-Natta polymerization.
References
- https://testbook.com/chemistry/ziegler-natta-catalyst
- https://www.vedantu.com/chemistry/ziegler-natta-catalyst
- https://unacademy.com/content/neet-ug/study-material/chemistry/ziegler-natta-catalyst/
- https://collegedunia.com/exams/ziegler-natta-catalyst-chemistry-articleid-7068
- https://www.britannica.com/science/Ziegler-Natta-catalyst
- https://www.vedantu.com/chemistry/ziegler-natta-catalyst
