Woodward hydroxylation reaction involves the synthesis of syn-diols or cis-diols from alkenes by hydroxylation.
A modified version of the Prevost hydroxylation process, the Woodward hydroxylation reaction includes treating alkenes with iodine and silver acetate in the presence of water to produce cis-diols or cis-glycols. Robert Burns Woodward, an American organic chemist, first explained this reaction in 1954.
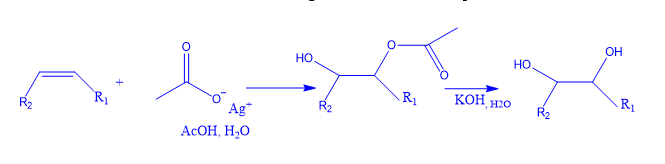
An alkene treated with iodine and silver acetate will experience nucleophilic displacement with acetate in the presence of water. The intermediate product undergoes hydrolysis to produce the necessary diols.
The twin-OH groups, or two hydroxy groups adjacent to the carbons, are known as “Syn-diols/cys-diols.” The desired form of diols would emerge from the displacement of the nucleophile.
An SN2 displacement, which occurs when one of the double bond’s two bonds is displaced by a backside attack with the stereochemistry and can be challenging in some circumstances, is comparable to the Woodward reaction of an anion with a double bond.
Interesting Science Videos
Reaction mechanism of Woodward hydroxylation reaction
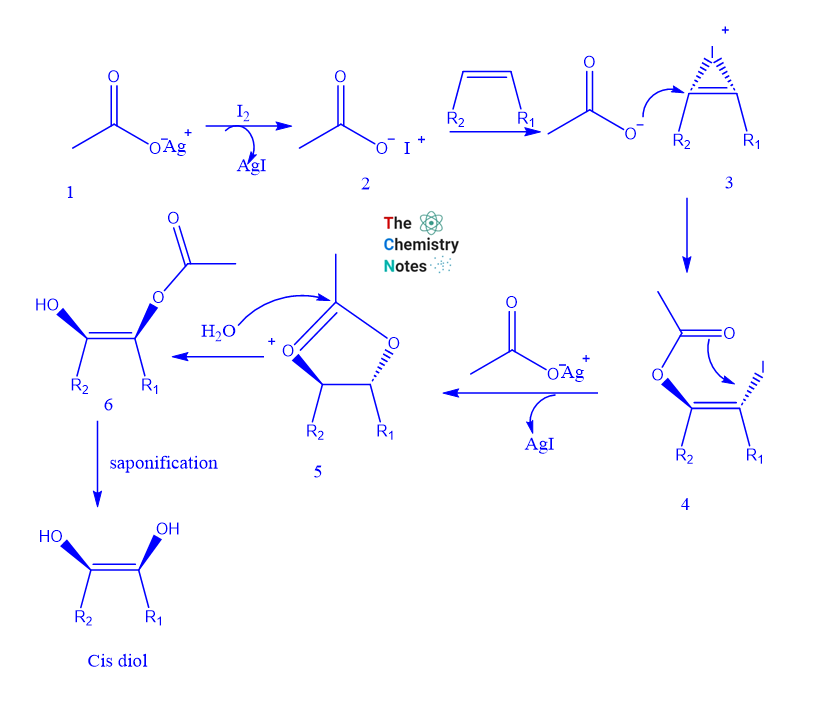
- The Woodward reagent is an equimolar solution of iodine and silver acetate or silver benzoate in wet acetic acid.
- Using this reagent alkenes are changed into cis-1,2 diols.
- An olefin reacts with iodine in the presence of silver acetate to produce an iodonium ion, which is then replaced by an acetate ion in an SN2 reaction to produce trans-iodoacetate. o Anchimeric support from the acetate group combined with the strong bonding ability of the silver ion for iodine results in a cyclic acetoxonium ion.
- In moist conditions, the acetoxonium ion traps water and reacts to produce a cis-hydroxyacetate, which, after saponification produces cis-diols.
The silver acetate aids in the iodine’s interaction with the alkene, resulting in the formation of the iodonium ion (3). Acetic acid (or silver acetate) opens the iodonium ion via the SN2 reaction to produce the first intermediate, iodoacetate (4).
With the help of anchimeric assistance, the iodine is removed through a second SN2 reaction to produce an oxonium ion (5), which is then hydrolyzed to produce the monoester (6).
Applications of Woodward hydroxylation reaction
- The Woodward reaction is utilized in organic chemistry and steroid chemistry.
- It is used to produce long-chain olefinic chemicals.
- The production of cis-diols from alkenes is the reaction’s main synthetic benefit.
- Additionally, because this reaction is stereoselective, it can be utilized for the synthesis of natural compounds, colors, medications, and other things.
References
- https://byjus.com/chemistry/woodward-reaction/.
- https://www.organic-chemistry.org/namedreactions/woodward-reaction.shtm.
- Morrison, R. T., & Boyd, R. N. (1983). Organic chemistry. Boston: Allyn and Bacon.
- Sthapit, M. K., Pradhananga, R. R., Bajracharya, K. B., (2014). Foundations of chemistry. Taleju Prakashan.
- Arun Bahl, B.S. Bahl and G.D. Tuli. (1999). Study Guide and Solutions Manual For : Essentials of Physical Chemistry (1). New delhi: S. CHAND.
