Tungsten is a chemical element with the atomic number 74 and it is represented by the symbol ‘W’ in the periodic table. It is hard and brittle with a lustrous greyish-white appearance and belongs to the d-block of the periodic table. It is classified as a transition metal. It is a rare metal that occurs nearly entirely naturally on Earth as compounds with other metals. Tungsten chemical compounds are formed in oxidation levels ranging from -II to VI.
Interesting Science Videos
History of Tungsten
- In 1779, Irish scientist Peter Wolfe discovered tungsten through his investigation of the mineral wolframite.
- Carl W. Scheele extracted tungsten as tungstic oxide (WO3) from the mineral scheelite (calcium tungstate) in Sweden in 1781. However, he lacked a sufficient furnace for reducing the oxide to metal.
- Fausto and Juan Jose de Elhuyar, brothers from Spain, successfully recovered tungsten in 1783 by reducing acidified wolframite with charcoal.
- The element’s name is derived from the Swedish term ‘tung sten‘, which means heavy stone. The chemical symbol W is derived from the element’s original name, Wolfram.
Occurrence of Tungsten
- Tungsten has yet to be discovered in its natural condition. However, tungsten is found predominantly in minerals such as wolframite and scheelite.
- The minerals ferberite (FeWO4) and hübnerite (MnWO4) combine to form wolframite.
- The mineral scheelite is calcium tungstate (CaWO4). The commercial worth of various tungsten minerals ranges from low to extremely uncommon and virtually nonexistent.
- It can be extracted commercially through the reduction of tungsten oxide with carbon or hydrogen. It is mined in China (the world’s largest producer), Austria, Portugal, and Bolivia, among other places.
- Tungsten has 33 isotopes having known half-lives and mass numbers ranging from 158W to 190W. Tungsten occurs naturally as a number of five isotopes.
Isotopes of Tungsten
Tungsten is found naturally on Earth in the form of five isotopes, namely 180W, 182W, 183W, 184W, and 186W.
| Isotopes | Natural Abundance (% atoms) |
|---|---|
| 180W | 0.12 (1) |
| 182W | 26.50 (16) |
| 183W | 14.31 (4) |
| 184W | 30.64 (2) |
| 186W | 28.43 (19) |
Elemental Properties of Tungsten
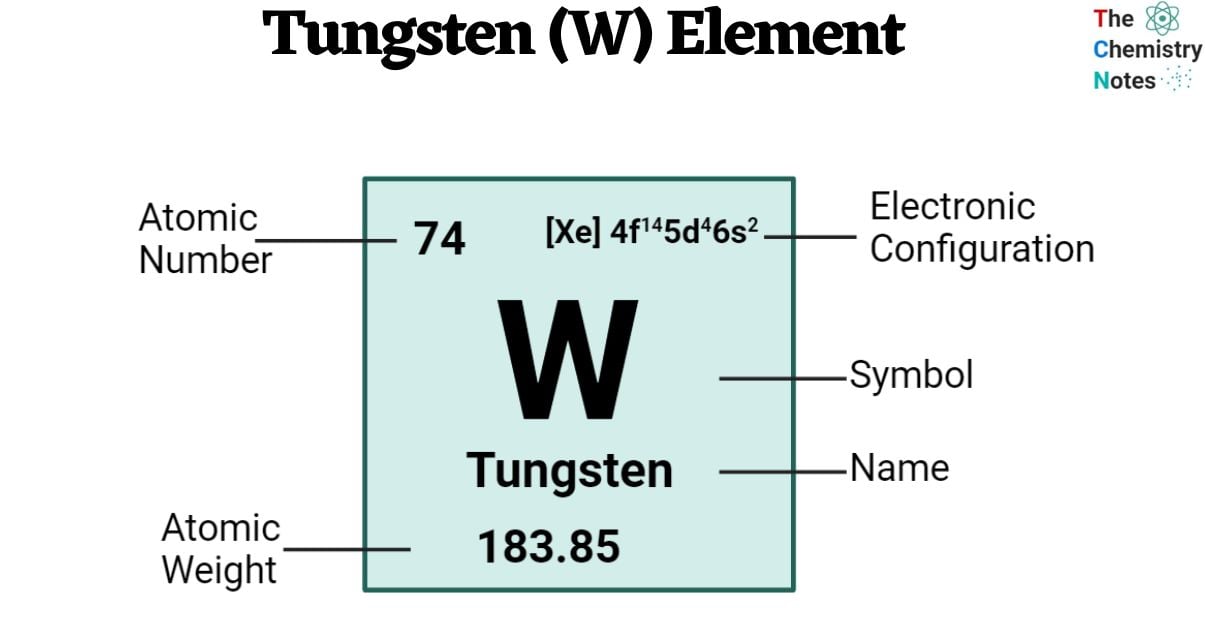
| Electronic Configuration | [Xe] 4f14 5d4 6s2 |
| Atomic Number | 74 |
| Atomic Weight | 183.85 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 6, 6, d-block |
| Density | 19.3 g.cm-3 at 20 °C |
| Appearance | greyish-white |
| Van der Waals radius | 0.137 nm |
| Electron shells | 2, 8, 18, 32, 12, 2 |
| Electrons | 74 |
| Protons | 74 |
| Neutrons in the most abundant isotope | 110 |
Physical Properties of Tungsten
- Tungsten has an atomic number of 74 and is a greyish-white rare earth metal. It has a melting point of 3422°C (6192°F) and a boiling point of 5930°C (10706 °F).
- Tungsten has a solid phase density of 19.25 g/cm3 and a liquid or molten phase density of 17.6 g/cm3.
- Brittle tungsten is difficult to deal with, but as it is purified, it becomes malleable and hence simpler to work with.
- Among all metals in pure form, tungsten has the highest tensile strength and melting point, as well as the lowest vapor pressure at temperatures over 1650°C. It also boasts the smallest thermal expansion coefficient of any pure metal.
- Tungsten appears primarily in two forms: one stable with a body-centered-cubic structure and the other metastable.
| Color/physical appearance | metallic, greyish-white |
| Melting point/freezing point | 3695K (3422°C, 6192°F) |
| Boiling point | 6203 K (5930 °C, 10706 °F) |
| Density | 19.25 g/cm3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 2.36 (Pauling Scale) |
Chemical Properties of Tungsten
- It is resistant to the majority of acids and bases.
- It combines with oxygen to generate a tri-oxide at high ‘red-hot’ temperatures.
- In nature, tungsten metal is usually non-reactive.
- It does not react with water, oxygen, or air at ambient temperature.
- At normal temperature, tungsten (VI) fluoride is easily formed by reacting with fluorine.
- It is the sole element in the third transition series known to appear in biomolecules.
- It reacts with bromine and chlorine at 250°C.
Chemical Reaction of Tungsten
- The Reaction of Tungsten with Air
Tungsten does not react with oxygen or air when it is at room temperature. The trioxide tungsten (VI) oxide, WO3, is produced at high temperatures (red heat). Tungsten metal that has been finely split is pyrophoric.
Δ
2 W(s) + 3 O2(g) → 2 WO3(s)- The Reaction of Tungsten with Water
Under normal circumstances, tungsten does not react with water.
- The Reaction of Tungsten with Halogens
In a direct reaction, tungsten forms tungsten(VI) fluoride, WF6, with fluorine, F2, at ambient temperature.
W (s) + 3 F2 (g) → WF6 (g) [colorless]At 250°C, tungsten combines immediately with chlorine, Cl2, to generate tungsten(VI) chloride, WCl6.
Δ
W (s) + 3 Cl2 (g) → WCl6 (s) [dark blue]At 250°C, tungsten combines immediately with bromine, Br2, to generate tungsten(VI) bromide, WBr6.
when heated Δ
W (s) + 3 Br2 (l) → WBr6 (s) [dark blue]
When tungsten metal and chlorine, Cl2, react under precisely regulated circumstances, tungsten(V) chloride, WCl5, is created.
Δ
2W (s) + 5 Cl2 (g) → 2 WCl5 (s) [dark green]It appears that tungsten and iodine, I2, do interact to some extent at red heat.
Δ
W (s) + 3 I2 (g) → WI6 (s)- The Reaction of Tungsten with Acids
Under normal circumstances, tungsten does not react with most acids.
Uses of Tungsten
- Tungsten is a valuable metal that is commonly utilized in light bulb filaments, electron and television tubes, abrasives, and specific alloys like steel tools.
- Owing to tungsten’s capacity to retain strength at high temperatures and its extremely high melting point, it has multiple uses in the area of electronics, involving incandescent lights, vacuum tube filaments, cathode-ray tubes, heating elements, integrated circuits, field emission guns, nanoelectronics, and so on.
- Tungsten Carbide is crucial in the mining and petroleum sectors. As a result, contamination from these sources is conceivable in both industrial and urban regions.
- X-ray screens employ calcium and magnesium tungstate phosphors to convert X-rays into blue visible light, and medical X-ray tubes use tungsten emitter coils.
- Tungsten has a density that is quite close to that of gold, hence it is frequently used as a gold replacement. It has also been used as a platinum replacement.
- Tungsten is also employed in microprocessor and liquid crystal display technologies.
- Tungsten alloyed with other metals possesses advantageous uses in the military, where it has been employed in bullets, grenades, shells, and missiles.
Health Effects of Tungsten
- All tungsten compounds should be considered very hazardous. Metal dust is a fire and explosive danger.
- This substance has not been shown to worsen medical issues when exposed repeatedly or for an extended period of time.
- Acute health consequences include irritation of the skin and eyes upon contact. Inhalation will irritate the lungs and mucous membranes. Irritation of the eyes causes watering and redness. Skin inflammation is distinguished by reddening, scaling, and itching.
Environmental Effects of Tungsten
- Tungsten is not believed to be environmentally dangerous. There are no particular data on ecotoxicity.
Video References
References
- https://www.chemistrylearner.com/tungsten.html
- https://www.britannica.com/science/tungsten-chemical-element
- https://education.jlab.org/itselemental/ele074.html
- https://www.chemicool.com/elements/tungsten.html
- https://www.lenntech.com/periodic/elements/w.htm
- https://chemicalengineeringworld.com/tungsten-element-properties-and-information/
- https://www.webelements.com/tungsten/chemistry.html
- https://pilgaardelements.com/Tungsten/Reactions.htm

