Tin is a metallic element with the atomic number 50 and is represented by the symbol ‘Sn’ in the periodic table. It is classified as a post-transition metal and belongs to the p-block of group 14 of the periodic table. It has a silver metallic luster with a hint of a bluish tinge. Tin has chemical properties comparable to those of its group 14 neighbors, germanium and lead. It is the 49th most abundant element on Earth’s crust consisting of 2.3 (ppm) parts per million. The mineral cassiterite, which includes stannic oxide (SnO2), is the primary source from which it is extracted.
Tin has two primary allotropes: α-tin (grey) and β-tin (white); beta-tin is more stable, silvery-white in appearance, and malleable at room temperature. However, alpha-tin is more stable at lower temperatures and has a diamond-cubic structure. It has two primary oxidation states :+2 and the relatively more stable +4. It has ten naturally occurring stable isotopes (the highest number in the periodic table).
Interesting Science Videos
History of Tin
- The discovery of tin has long been lost to ancient civilizations. However, the first use dates back to the Bronze Age, around 3000 B.C.
- The mining of tin in England and Spain was at an all-time high during the 8th century BCE and the 6th century.
- British scientist Robert Boyle performed experiments on the tin and published a paper describing the oxidation of the element in 1673, which further increased the experimental studies.
- Element gets its name from the Anglo-Saxon word “tin”, the symbol ‘Sn’ derives from the Latin word ‘stannum’ meaning ‘tin’.
Occurrence Of Tin
Tin is the 49th most abundant element on Earth’s crust consisting of 2.3 (ppm) parts per million. It does not occur freely in the nature so it is extracted from various ores. Tin occurs in different ores and minerals, some of which are discussed here:
- Cassiterite: It is the primary ore mineral of tin, and it is a generally recognized and abundant tin-bearing mineral. It can be extracted from cassiterite using a process called cassiterite extraction. Cassiterite is a type of tin oxide mineral (SnO2) that has a very high specific gravity and a color variation that ranges from brownish-black to black in appearance.
- Stannite: It is a sulfide mineral that is relatively complex and comprises tin, copper, iron, and zinc in its foundation. It usually appears as metallic crystals that can vary in color from gray to black, and its chemical formula is (Cu, Fe, Zn)2SnS4.
- Tin-bearing Sulfides: Some sulfide minerals, including sulfosalts and sulfides, have the potential to have trace amounts of tin as one of their constituents. Cylindrite (Pb3Sn4FeSb2S14), Franckeite (Pb5Sn3Sb2S14), and Teallite (PbSnS2) are all examples of minerals that fall into this category.
- Tin-bearing Feldspars: It’s possible for certain feldspar minerals, such as microcline and orthoclase, to have trace amounts of tin mixed in with them as an impurity. These tin-bearing feldspars are found in granitic rocks, but their impact on the production of tin is not as substantial as that of cassiterite or stannite.
- Tin-bearing Oxides and Hydroxides: Tin can also be found in the form of oxides and hydroxides, such as tin-bearing hematite (Fe2O3), tin-bearing rutile (TiO2), and tin-bearing brannerite (UO2) (Ti, Fe)2O6, which are all examples of tin-bearing oxides and hydroxides.
Some of the key producers of tin globally include China, Brazil, Peru, Bolivia, Russia, and Indonesia, among others.
Isotopes of Tin
Tin has the most naturally occurring stable isotopes in the periodic table. These stable isotopes include 112Sn, 114Sn, 115Sn, 116Sn, 117Sn, 118Sn, 119Sn, 120Sn, 122Sn, and 124Sn.
Naturally Occurring Stable Isotopes Of Tin
| Isotopes | Natural abundance (atom %) |
|---|---|
| 112Sn | 0.97 (1) |
| 114Sn | 0.66 (1) |
| 115Sn | 0.34 (1) |
| 116Sn | 14.54 (9) |
| 117Sn | 7.68 (7) |
| 118Sn | 24.22 (9) |
| 119Sn | 8.59 (4) |
| 120Sn | 32.58 (9) |
| 122Sn | 4.63 (3) |
| 124Sn | 5.79 (5) |
Elemental Properties of Tin
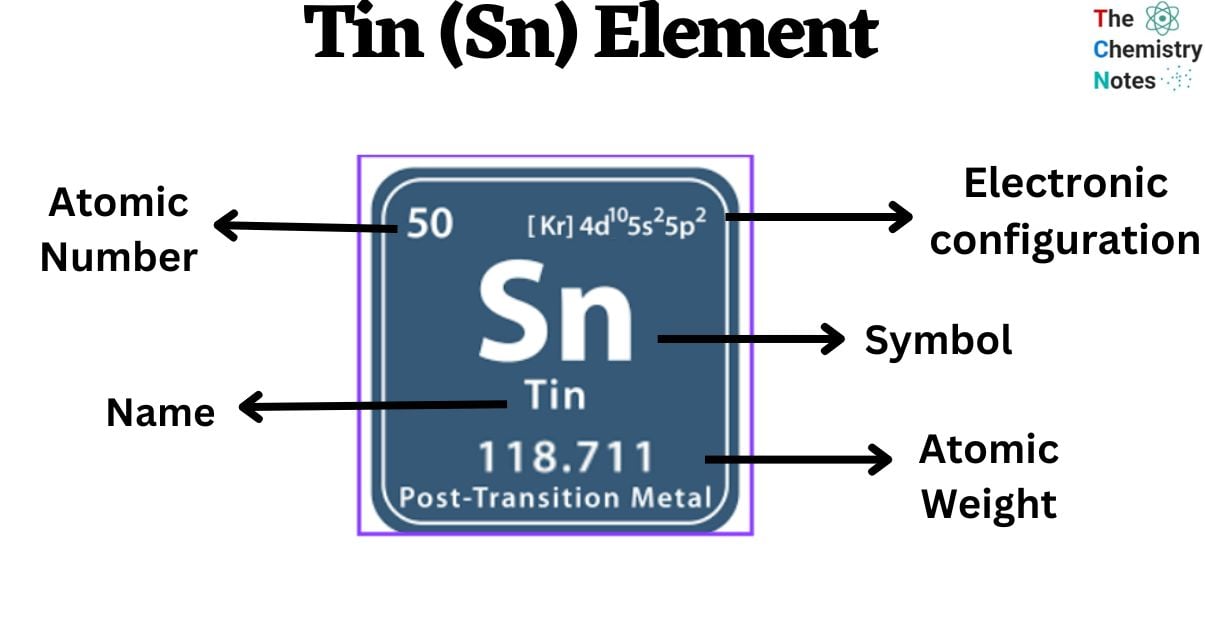
| Electronic Configuration | [Kr] 4d10 5s2 5p2 |
| Atomic Number | 50 |
| Atomic Weight | 118.71 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 14, 5, p-block |
| Density | white, β: 7.265 g/cm3 at 20 °C gray, α: 5.769 g/cm3 at 20 °C |
| Van der Waals radius | 0.162 nm |
| Electron shells | 2, 8, 18, 18, 4 |
| Electrons | 48 |
| Protons | 50 |
| Neutrons in most abundant isotope | 69 |
Physical Properties Of Tin
- Tin has an atomic number of 50 and is a lustrous silvery-white metal with a bluish tinge. White tin has a melting point of 232°C (450°F) and a boiling point of 2,260°C (4,100°F).
- Tin has two primary allotropes: α-tin (grey) and β-tin (white); white β has a solid phase density of 7.265 g/cm3, whereas grey α has a solid phase density of 5.769 g/cm3. Tin has a liquid or molten phase density of 6.99 g/cm3.
- It is soft which allows it to cut with a knife and its malleable property allows it to be easily hit into sheets without cleavage.
- β-tin or white tin also has a ductile property that allows it be to drawn into thin wires without breaking them however when temperature elevate above 200 °C it becomes brittle.
- Tin serves as a good electrical conductor. Because electrons in tin are free to move around they are able to carry electrical charge from one end to other.
- Tin is a good thermal conductor as well. Heat causes its particles to vibrate more rapidly and move around more swiftly. Energy is transferred from one particle to another as they come into contact.
- Tin’s crystalline structure manifests in three distinct forms, or “allotropes,” rather than in a single form. These three allotropic forms of tin are known as cubic, tetragonal, and rhombic tin. At room temperature, it exists as white tin but below 13 °C it changes into grey tin.
- Grey tin is non metallic because of its covalent structure.
- It is corrosion resistance.
- “Tin cry” is the word used for the screeching sound tin produces when it is bent. This is a bizarre property of tin.
| Color/physical appearance | Lustrous, silver-white, bluish tinge |
| Melting point/freezing point | 505.08 K (231.93 °C, 449.47 °F) |
| Boiling point | 2875 K (2602 °C, 4716 °F) |
| Density | white, β: 7.265 g/cm3 at 20° gray, α: 5.769 g/cm3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.96 (Pauling Scale) |
Chemical Properties Of Tin
- At room temperature, tin is relatively unreactive with both water and oxygen. But at elevated temperatures, it reacts with both water in the form of steam and oxygen to form tin oxide.
- Tin does not readily oxidizes however upon heating with concentrated nitric acid (HNO3) it oxidizes.
- It has relatively low toxicity but most compounds of tin are toxic.
- It has two primary oxidation states :+2 and the relatively more stable +4.
- It can be alloyed with other metals.
- Tin reacts with halogens to form compounds.
- It does not reacts rapidly with acids.
Chemical Reactions Of Tin
Here, we’ll look at how tin interacts with various substances.
- The Reaction of Tin With Water
While tin is stable in water at room temperature, it interacts with water to produce tin dioxide, SnO2, and hydrogen when heated with steam.
Sn (s) + 2 H2O (g) → SnO2 (s) + 2 H2 (g)
- The Reaction of Tin With Air
At room temperature, tin does not react with oxygen; however, when the temperature is elevated, tin reacts with oxygen to form tin dioxide, SnO2.
Sn (s) + O2 (g) → SnO2 (s)
- The Reaction of Tin With Halogen
When heated tin reacts with chlorine to generate tin (IV) chloride.
Sn (s) + 2 CI2 (g) → SnCI4 (s)
- The Reaction of Tin With Acids
With diluted hydrochloric acid (HCl), tin reacts slowly, but it reacts rapidly with concentrated acid to produce tin (II) chloride.
Sn (s) + 2 HCI (aq) → SnCI2 (s) + H2 (g)
- The Reaction of Tin With Alkali
hexahydroxystearate (IV) salts and hydrogen are produced when tin dissolves in strong alkali solutions.
Sn (s) + 2 NaOH (I) + H2O (I) → Na2SnO3 (aq) + 2 H2 (g)
Uses of Tin
Tin is a non-toxic element and was used in ancient history by humans but it is not used widely in modern-day industries. Some of the applications for tin are included here:
- Tin plating is used by ancient civilizations. This technique is still being used in the modern era. Different industries which include electronics, solar, mechatronics, hardware, and many more, use tin plating for several purposes. For example, screws, nuts, and bolts used in naval vessels; baking sheets and bowls used in cookware; terminals and housing used in avionics are all made by tin plating.

- Custom worktops made from tin sheets can be installed in home kitchens and bathrooms as well as in industrial settings that require corrosion resistance and durability for workplaces, break rooms, and dining areas. Tin sheets can also be utilized in both contexts.
- Tin sheets are frequently stamped and shaped into custom tin tiles in luxury homes. These tiles are then used to build custom walls and ceilings, giving the interior a look and feel that is truly one of a kind. Depending on the chemical makeup of the sheet metal, the tin sheet may also be produced in different colors for your purchasing convenience.
- Tin is frequently utilized in jewelry, particularly for individualized trinkets and pendants. This is possible since it is simple to cut thin sheets of tin into different shapes, such as circles, stars, and diamonds, and then stamp them with detailed designs. After that, you can make one-of-a-kind jewelry by attaching these pendants, charms, and trinkets to necklaces, earring posts and loops, and bracelets.
- Tin is utilized in a wide variety of manufacturing settings to assist in the production of final items. For instance, window glass is produced by allowing molten glass to float on top of molten tin to provide a smooth and level surface. The method in question is known as the Pilkington procedure.
Health Effects of Tin
Tin is primarily used in a variety of biological materials. The most hazardous types of tin for people are the organic tin bonds. Despite the risks, they are used in numerous industries, including the paint and plastics industries, and agriculture through insecticides. Humans can uptake tin bonds through diet, air or skin. This ingestion of tin bonds can result in both acute and chronic effects.
Some of the health effects of tin are included here:
- Eye and skin irritations, Headaches, Indigestion, Nausea, vertigo, Excessive perspiration, Shortness of breath, Urination issues are some of the acute effects of tin on human health.
- Long-term consequences of tin on human health include depression, Liver impairment, Immune system dysfunction, Chromosomal damage, Insufficiency of red blood cells, Brain damage (resulting in rage, insomnia, amnesia, and migraines).
Environmental Effects of Tin
Tin as individual particles or atoms is not exceedingly lethal to living organisms; it is only toxic in its organic form. Since it is not biodegradable, organic tin can last longer in the environment. Microorganisms typically face difficulties when attempting to degrade tin compounds that have accumulated on water sediments for an extended period.
- Adsorbed onto sludge particles, organic containers can diffuse through water surfaces. They are exceedingly lethal to algae, fungi, and phytoplankton and are known to cause significant harm to aquatic organisms. There are numerous organic tins, and their toxicity levels vary considerably. Tributyltin is the most toxic tin element to fish and marine vegetation, whereas tributyltin is significantly toxic to phytoplankton.
Video on Tin
References
- https://pubchem.ncbi.nlm.nih.gov/element/Tin
- https://www.britannica.com/science/tin
- https://www.rsc.org/periodic-table/element/50/tin
- https://byjus.com/chemistry/tin/
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1150–151. ISBN 978-0-08-037941-8.
- https://study.com/academy/lesson/what-is-the-element-tin-used-for-lesson-for-kids.html
- John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Edition, 2011.

