Surface chemistry is a specialized branch of chemistry that focuses on the study of the interactions between a specific substance and the surface of a solid material.
Surface chemistry is a branch of science that focuses on investigating the various phenomena that take place at the interfaces or surfaces of substances. These phenomena include Adsorption, heterogeneous catalysis, the formation of colloids, corrosion, crystallization, dissolution, electrode processes, and Chromatography, among others.
Surface chemistry plays a crucial role in a wide range of applications, including analytical work, the medicinal field, and the paint industry, among others. Surface chemistry is a branch of science that focuses on the investigation of chemical reactions occurring at the boundary between two surfaces. These surfaces can encompass various combinations, such as solid-liquid, solid-gas, solid-vacuum, and liquid-gas interfaces, among others. Surface engineering encompasses various applications of surface chemistry.
Interesting Science Videos
Several Phenomena in Surface Chemistry
There exist a multitude of phenomena that manifest on the surface of substances, encompassing
- Adsorption
- Heterogeneous Catalysis
- Corrosion
- Crystallization
Adsorption
- Adsorption happens when there are unbalanced forces, which happen when crystals solidify or when there are unpaired electrons or empty valence orbitals in solids with d-orbitals. The phenomenon observed in liquids can be attributed to the presence of surface tension.
- Several examples, such as H2, N2, and O2, exhibit adsorption on the surface of activated charcoal.
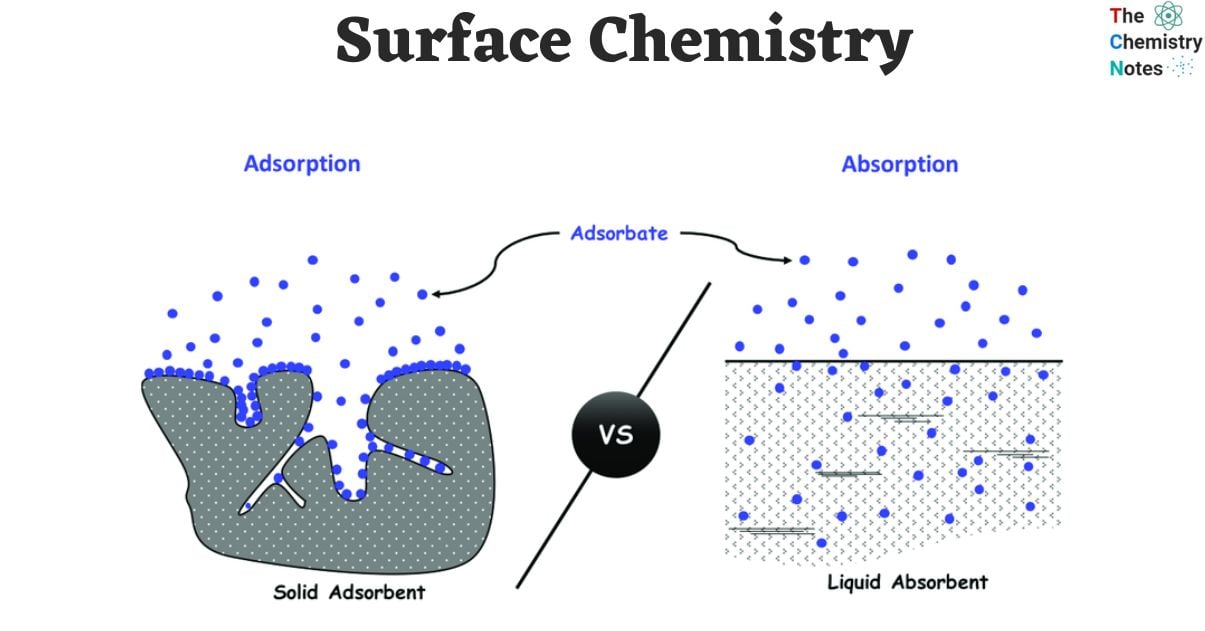
- The substance onto which the phenomenon of adsorption occurs is referred to as the adsorbent, whereas the molecular entities that gather on the surface are referred to as the adsorbate.
- The enthalpy of adsorption refers to the energy released when one mole of gas is adsorbed.
Difference Between Absorption and Adsorption
| Absorption | Adsorption |
|---|---|
| It is a bulk phenomenon. In absorption the substance penetrates into the bulk of the other substance. | Adsorption is a surface phenomenon. The adsorbing substance is called adsorbate and is only concentrated on the surface of the adsorbent. |
| Absorption occurs at a uniform rate. | The rate of adsorption is rapid to start with, and its rate slowly decreases. |
Adsorption in Terms of Gibb’s Helmholtz Equation
- The process of adsorption is characterized by an exothermic reaction. Hence, the liberation of energy, denoted as ΔH takes place concomitantly with adsorption. Consistently exhibits a pessimistic outlook and demonstrates a bias towards the process.
- Additionally, the molecules being adsorbed experience limited mobility on the surface of the adsorbent. Therefore, the entropy factor acts in opposition to the process.
- Based on Gibb’s Helmholtz Equation, the change in Gibbs free energy (ΔG) can be calculated by subtracting the product of temperature (T) and the change in entropy (ΔS) from the change in enthalpy (ΔH).
Δ G = Δ H - T Δ S- Given that adsorption indeed occurs, it can be concluded that the value of H is greater than T.
The value of G is negative. - As the process of adsorption progresses, the disparity between the two opposing tendencies gradually diminishes until they reach a state of equilibrium.
i.e., Δ H = T Δ S, or Δ G = 0- At this stage, equilibrium, called adsorption equilibrium, is established. and there is no net adsorption taking place at this stage.
Types of Adsorption
The comprehension of the nature of forces that exist between the adsorbate and adsorbent molecules enables us to categorize the adsorption process into two distinct types.
Physical Adsorption / Vander Waal’s Adsorption/ Physisorption
- Physical adsorption is a complex process that involves multiple layers, where a weak van der Waals force acts between the adsorbent and the adsorbate.
- The process under consideration is reversible and demonstrates a low activation energy. Its behavior is primarily influenced by factors such as the nature of the adsorbate, temperature, and pressure.
- The substance exhibits a relatively low enthalpy of adsorption, typically ranging from 20 to 40 KJ/mol.
Chemical Adsorption / Chemisorption
- Chemical adsorption is a phenomenon that involves the creation of a monolayer of molecules on the surface of adsorbent material.
- This process is primarily driven by the presence of robust chemical forces between the adsorbent and the adsorbate.
- Chemisorption is irreversible and necessitates a substantial amount of activation energy.
- The outcome of the process is predominantly influenced by the temperature of the adsorbate and the surface area of the adsorbent.
- The substance demonstrates a notable enthalpy of adsorption, with values ranging from 40-400 KJ/mol.
Difference Between Chemisorption and Physisorption
| Chemisorption | Physisorption |
|---|---|
| The process of adsorption is driven by chemical forces, specifically covalent or ionic bonding. | The process of adsorption is primarily driven by the interaction of weak van der Waals forces. |
| A monomolecular layer will be created. | has the potential to create multimolecular layer. |
| The process is irreversible. | The process can be readily reversed. |
| The molecular state has the potential to exhibit variation. Surface chemicals are present. | The molecular state of the adsorbate on the adsorbent remains unchanged. There is an absence of surface chemicals. |
| The phenomenon initially manifests at elevated temperatures, but thereafter exhibits a decline. | The phenomenon typically manifests swiftly under low-temperature conditions and diminishes as the temperature rises. |
| The impact of pressure variation on chemisorption is somewhat diminished. | The observed phenomenon has a positive correlation with an increase in pressure. |
| There is no discernible correlation between the degree of adsorption and the level of easiness in the process of gas liquefaction. For example, The adsorption of hydrogen (H2) on platinum (Pt), the decomposition of ammonia (NH3) in the presence of tungsten, and the decomposition of hydrogen iodide (HI) on gold (zero order) are the topics of interest. | The extent of adsorption is contingent upon the ease of liquefaction, which is determined by factors such as surface area, critical temperature, and inversion temperature. The constant ‘a’ in Vanderwaal’s equation, for example. The phenomenon of gases being adsorbed onto charcoal. |
| Chemisorption requires a significant amount of external activation energy. | The presence of external activation energy is unnecessary. |
| The enthalpy of adsorption is high. | The enthalpy of adsorption is low. |
Applications of Adsorption
There are several uses of adsorption some of which are discussed here:
Used in Gas Mask
- In the context of gas masks, the utilization of divided coconut charcoal is implemented as an absorbent substance to efficiently capture and neutralize perilous gases, including methane (CH4), carbon monoxide (CO), and phosgene (COCl2). Commonly utilized in coal mines, its primary function is to absorb noxious gases during the process of respiration.
Used in Vaccums
- Activated charcoal is intentionally inserted into the walls of Dewar flasks to effectively adsorb any gas that may come into contact with the annular space.
- The occurrence of gas infiltration can arise from inherent defects in the glass structure or through the process of diffusion across the glass substrate.
Used in Sugar Clarification
- The process of decolorizing sugar involves the treatment of the sugar solution with charcoal powder. The latter substance selectively absorbs the unwanted hues that are present.
Used as Heterogeneous catalysis
- The acceleration of the reaction rate is facilitated by the process of adsorbing reactant molecules onto the solid surface of the catalysts.
- Many practical and significant gaseous reactions involve solid materials. Catalysts are of paramount importance in a wide range of chemical reactions.
- Two notable industrial processes include the synthesis of ammonia utilizing iron as a catalyst and the creation of sulfuric acid (H2SO4) via the contact process.
- The application of finely divided nickel in the process of oil hydrogenation serves as a prominent example of heterogeneous catalysis.
Used In Chromatography
- A range of chromatographic techniques, such as adsorption, paper, and Column Chromatography, are utilized for the purification and separation of chemicals that are present in low quantities.
- These techniques are founded on the principle of selective adsorption.
Adsorption Isotherm
The relationship between the amount of gas adsorbed by an adsorbent and the pressure, while keeping the temperature constant, can be described using a graphical representation known as an adsorption isotherm.
Freundlich Adsorption Isotherm
The Freundlich adsorption isotherm is a widely recognized empirical correlation that characterizes the association between the quantity of gas adsorbed per unit mass of solid adsorbent and the pressure at a given temperature. The mathematical expression for this can be represented as follows:
x/m = k. p (1/2) (n > 1)Where,
- x = mass of the gas adsorbed on the solid adsorbent
- m = mass of solid adsorbent
- p = pressure
- k & n = Constants that depend on the following:
- Nature of the adsorbent
- Nature of the gas at a specific temperature
Logarithmic Equation of Freundlich Adsorption Isotherm
log (x/m) = log k + (1/n) log pIf, (1/n) = 0, (x/m) = constant then the adsorption is independent of pressure.If, (1/n) = 1, (x/m)p, the adsorption varies directly with pressure.Catalysis
- A catalyst is a substance that modifies the rate of a chemical reaction while generally maintaining its original form and being fully recoverable once the process is through. Nevertheless, it is conceivable for the entity to engage in a chemical process wherever it is utilized in a particular phase and subsequently restored in a subsequent stage. The phenomenon that has been observed is widely known as catalysis.
- A substance is referred to as a positive catalyst, or simply as a catalyst if it enhances the pace of a chemical process. Conversely, the introduced substance is referred to as a negative catalyst when it hinders the rate of a chemical process.
- For instance, In the process of converting hydrogen peroxide into water and oxygen gas, potassium permanganate serves as a catalyst, effectively enhancing the reaction rate.
- 2H2O2 Potassium permanganate→ 2 H2O + O2
Types of Catalysis
Catalysis can be categorized into two distinct categories, which are determined by the phases of both the catalysts and reactants that are involved.
- Homogeneous Catalysis
- Heterogeneous Catalysis
Homogeneous Catalysis
- When the catalyst is in the same phase as the reactants, it is referred to as a homogeneous catalyst. This particular form of catalysis is known as homogeneous catalysis.
Examples of Homogeneous Catalysis and Catalysts
Hydrolysis of Sugar
- The process of sugar breakdown includes the utilization of a sucrose solution and water as reactants, both of which exist in liquid states.
- Furthermore, the utilization of the liquid catalyst known as sulfuric acid (H2SO4) is employed in this particular method.
The subsequent depiction illustrates the reaction.
H2SO4
C12H22O11 (aq) + H2O (l) H2SO4 (l) → C6H12O6 (aq) + C6H12O6 (aq)
Sucrose Glucose Fructose
Hydrolysis of the Ester
- During the hydrolysis process, the ester is combined with fluid water to initiate the reaction.
- This reaction takes place in the presence of a catalyst, hydrochloric acid (HCl), which is also in a liquid state.
The following is the representation of the reaction.
HCl (l)
CH3COOCH3 (l) + H2O (l) → CH3COOH (aq) CH3OH (aq)Heterogeneous Catalysis
- In this particular form of catalysis, the catalyst is situated in a distinct phase in relation to the reactants.
- In the field of heterogeneous catalysis, it is common practice for the catalyst to be in a solid state, while the reactants are typically in a gaseous form.
- However, it is worth noting that there are instances where liquid reactants are employed. This phenomenon is widely recognized and referred to as surface catalysis.
Examples of Heterogeneous Catalysis and Catalyst
Synthesis of Ammonia
- The process of ammonia synthesis, as described by Haber’s method, entails the usage of gaseous nitrogen and hydrogen, in conjunction with a solid iron catalyst.
Fe
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g)Formation of Sulfuric Acid
- During the progression of this particular chemical reaction, the gaseous component sulfur dioxide experiences an oxidation phenomenon, leading to its conversion into gaseous sulfur trioxide.
- The conversion described above occurs through the process of heterogeneous catalysis, wherein a solid catalyst containing V2O5 is present. Subsequently, the process of hydrolysis is employed to convert sulfur trioxide into sulfuric acid.
V2O5(s)
SO2 (g) + O2 (g) → 2 SO3 (g)Enzyme
- Numerous enzymes also function as catalysts for a wide range of processes. Enzymes play a crucial role in facilitating several biochemical events that take place within the human body, as well as in plants and animals.
- Biochemical catalysts, sometimes referred to as enzymes, are recognized for their ability to accelerate chemical reactions inside biological systems. This process is commonly referred to as biochemical catalysis.
- Enzymes are characterized by their chemical composition, as they consist of globular proteins with a substantial molar mass that typically falls within the range of (15,000- 1,000,000) g mol-1.
- Furthermore, these enzymes have the ability to create colloidal solutions when dissolved in water.
Mechanism of Enzyme Catalysis
- Enzymes are referred to as biochemical catalysts. The mechanism associated with enzyme catalysis is commonly referred to as the lock and key mechanism. This mechanism can be described in the following steps:
- The first step in enzymatic processes involves the formation of a complex between the enzyme and substrate.
- During the second stage, the dissociation of the complex formed by the enzyme and substrate leads to the production of the product.
Properties of Enzymes
Enzymes possess several significant properties:
- Each enzyme is responsible for catalyzing a singular chemical reaction.
- Enzymes have a high degree of catalytic efficiency. They enhance the rate of a chemical reaction by a magnitude of up to 1020.
- Enzymes exhibit optimal efficiency only when they are present in limited amounts.
- Enzymatic reactions are characterized by their highest level of activity at a particular pH referred to as the optimum pH. This optimal pH value is frequently observed to be about 7.4 Furthermore, it is observed that these reactions exhibit optimal performance within a temperature range of 298-310K, while being exposed to air pressure of one unit.
- The enzymatic activity of specific enzymes is enhanced in the presence of particular compounds known as coenzymes. For instance, when a protein incorporates a limited quantity of vitamins as its non-protein component, its functionality is augmented. The activators often consist of metal ions such as sodium (Na+), copper (Cu+2), and manganese (Mn+2).
- Enzymatic activity is regulated by several methods and can be impeded by certain chemical compounds referred to as inhibitors, for example, drugs.
Colloids
- Colloids are characterized as heterogeneous mixes comprising two substances, wherein minuscule particles of one component are scattered within another substance.
- The substance that consists of minute particles suspended within another substance is referred to as the dispersed phase, whereas the substance in which it is suspended is known as the dispersion medium.
- In the context of fog dispersion, the phase of water is predominantly liquid, whereas the dispersion medium consists of various gases.
- The particles of the dispersed phase in colloids are not visible to the naked eye due to their minuscule size.
Properties of Colloids
Colloids exhibit a range of properties, which are outlined below:
- Colloids can be classified as heterogeneous mixtures.
- The particles of colloids exhibit a remarkably small size, typically falling within the range of (1-1000) nm.
- Colloidal suspensions exhibit the Tyndall effect.
- The feasibility of separating colloidal particles using filtration is limited, however, centrifugation can efficiently accomplish their separation.
- The phenomenon of Brownian motion is observed in colloidal particles.
- The particles present in a colloid demonstrate the property of sustained suspension, wherein they do not settle even after being left undisturbed for extended periods of time.
Classification of Colloids
- Colloids can be categorized into eight distinct types, which are determined by the physical state of both the dispersed phase and the dispersion medium.
The following is a compilation of the eight distinct categories of colloids:
| Name | Appearance | Dispered Phase | Dispersion Medium | e.g. alloys |
|---|---|---|---|---|
| Solid Sol | (solid) | Solid | Solid | Coloured glasses, Pearl, Ruby, alloys, gems |
| Sol | (liquid) | Solid | Liquid | Ag, Sol, Au, Sol., S. Sol., Muddy water, gelatin in water, paint |
| Aerosol | (gas) | Solid | Gas | Smoke, dust, strom |
| Emulsion | (liquid) | Liquid | Liquid | Milk, medicines, oil water, blood |
| Gel | (solid) | Liquid | Solid | shampoo, jelly, cheese, butter, all fruits and veg, curd |
| Aerosol of Liquid | (gas) | Liquid | Gas | Cloud, fog, mist, spray |
| Foam | (liquid) | Gas | Liquid | Soap leather, whipped cream, shaving cream, soda water, froath |
| Solid Foam | (solid) | Gas | Solid | Styrene foam, Foam rubber |
Classification Based on Nature of Interaction between Dispersed Phase and Dispersion Medium
- The nature of the interaction between the dispersed phase and dispersion medium is a crucial factor to consider.
- Colloids can be classified into two distinct types based on the nature of the interaction between the dispersed phase and the dispersion medium.
- Lyophilic sols
- Lyophobic sols
Lyophilic sols
- The term “lyophilic” refers to a substance or molecule that exhibits an affinity or attraction towards liquids. Lyophilic sols are characterized by a strong attraction between the dispersed phase and the dispersion medium, which is typically water.
- An illustration of a colloidal solution can be observed when starch is dissolved in water. The dispersion medium in this colloidal solution is water, while the dispersed phase consists of starch.
- The preparation of this solution involves heating water to a temperature of 100 °C and subsequently dissolving starch within it.
- The sol exhibits stability as a result of the significant intermolecular forces between the dispersed phase and the dispersion medium, rendering it resistant to separation.
- The egg albumin sol serves as an additional illustration of a lyophilic sol.
Lyophobic sols
- The term “lyophobic” refers to a substance or material that exhibits a strong aversion or repulsion towards liquids. In these solutions, particles in the dispersed phase exhibit minimal or no attraction toward the dispersion medium.
- These solutions lack stability and are susceptible to separation. When a small amount of electrolyte is introduced into these types of sols, the dispersed phase and dispersion medium can be readily separated.
- In order to enhance their stability, it is necessary to incorporate stabilizers during the preparation process. These substances are commonly referred to as hydrophobic sols.
- One example of a lyophobic sol is ferric hydroxide sol.
- The preparation of ferric chloride involves hydrolysis.
- Boiling water is employed in this process.
- During this process, the production of hydrochloric acid occurs, which is subsequently eliminated from the sol due to its destabilizing effect.
- The ferric hydroxide sol (lyophobic sol) is removed through the process of dialysis.
References
- https://byjus.com/jee/surface-chemistry/
- https://www.vedantu.com/chemistry/surface-chemistry
- https://testbook.com/chemistry/surface-chemistry
- https://.com/surface-chemistry/
- https://collegedunia.com/exams/surface-chemistry-chemistry-articleid-1907
- https://www.mlsu.ac.in/econtents/1210_surfacechemistrytutornotes-190628091806.pdf
