Metals are made up of atom aggregates that are consistently organized in a crystalline structure. Metals and alloys are typically crystalline in structure. Generally, pure metals have fairly basic crystal structures with cubic or hexagonal unit cells, as we will see. Alloy crystal formations, on the other hand, can be quite complex.
Introduction to Metals
Elements are classified into three types: metals, metalloids, and non-metals
Any group of substances distinguished by strong electrical and thermal conductivity, as well as malleability, ductility, and high light reflectance. A metal is a material with a lustrous look that conducts electricity and heat reasonably well when freshly manufactured, polished, or shattered. Metals are ductile (they may be formed into wires) and malleable (they can be hammered into thin sheets). These qualities are caused by the metallic link formed between the metal’s atoms or molecules.
Metals account for around three-quarters of all known chemical elements. Aluminum, iron, calcium, sodium, potassium, and magnesium are the most prevalent in the Earth’s crust. Although the great majority of metals are found in ores (mineral-bearing substances), a few, such as copper, gold, platinum, and silver, are usually found in their free state because they do not readily react with other elements.
Alloys
An alloy is a metallic-solid solution or mixture of two or more elements.
Alloys include metals including brass, pewter, phosphor bronze, amalgam, and steel. The microstructure of complete solid solution alloys is a single solid phase. Depending on the thermal history, partial solutions produce two or more phases that may or may not be homogenous in distribution. In general, the properties of an alloy are frequently distinct from those of its constituent elements.
Alloys include metals including brass, pewter, phosphor bronze, amalgam, and steel. The microstructure of complete solid solution alloys is a single solid phase. Depending on the thermal history, partial solutions produce two or more phases that may or may not be homogenous in distribution. The properties of an alloy are frequently distinct from those of its constituent elements.
Characteristics of Metals and Alloys
Metals are distinguished by their distinct properties. These are some examples:
- Extremely high melting point
- Excellent heat and electricity conductors
- malleable (easy to bend/shape)
- Ductile (can be stretched readily without damage) (can be stretched easily without breakage)
- Density is high.
Usually, alloys are created to “maximize” particular properties. The following are the distinctions between metals and alloys: Harder than component metals.
- More corrosion resistant than pure metals
- Lower melting point than component metals
- More ductile than component metals
- More durable than component metals
- Less conductive than component metals
Steel (iron + carbon) is a typical alloy used in building materials. This makes sense because it can hold more weight, is less likely to corrode, and can be molded more easily than iron.
Structure of Metals and Alloys
Aggregates of atoms that are regularly organized in a crystalline structure make up metals. In contrast to what we have discussed thus far, which is the production of single crystals, metals typically form from a myriad of tiny crystals rather than from a single solidification from the melt.
This occurs because the molten metal often contains a large number of crystallization nuclei that are dispersed. When four atoms are able to form a unit cell and lose enough thermal energy, such nuclei may arise. As more metal atoms get sufficiently low in energy to join in, these unit cells will expand, leading to the development of crystals. Homogeneous nucleation is the name given to this procedure.
More frequently, the presence of contaminants in the melt causes solidification to begin. Crystals start to form as the temperature falls below the melting point and as a result metal atoms start to accumulate on these impurities. Heterogeneous nucleation is the name given to this phenomenon. When all of the metal has formed, the crystals (also known as grains) will stop growing. They will start to collide as they expand, thus creating boundaries between the crystals where the atoms are placed erratically. This border, known as the grain boundary, is basically a defect in the metal’s crystal structure.
Atomic Structure of Metals and Alloys
The atoms in a metal lattice form a pattern that may be represented as a 3D box shape known as the unit cell, which repeats over the entire metal.
Alloys are distinct. The type of alloy determines the atomic structure: substitutional or interstitial.
In a substitutional alloy, the atoms of one metal are substituted with those of another. These new atoms are similar in size to the atoms of the other metals.
The atoms of the second metal in an interstitial alloy are substantially smaller than those of the pure, original metal. These smaller atoms fit into the original structure’s “holes.”
Crystal Structure of Metal and Alloys
Crystals are three-dimensional repeating patterns. The fundamental repeating unit of the crystal is called the unit cell. It is a three-dimensional shape that may be replicated indefinitely using unit translations to fill holes in the structure.
Usually, a crystal has one of three major structures:
- Body-centered cubic (Bcc)
- Hexagonal closed packed (Hcp)
- Cubic-closed packed (ccp)/face-centered cubic (fcc)
The hexagonal or rhombic unit cells of this pattern would be replaced in three dimensions by three-dimensional boxes that would stack together to occupy all space.
Since metal atoms are roughly shaped like spheres, they do not pack completely tightly and has some gaps in between the spheres. The packing efficiency of different unit cells varies. The number of atoms in the unit cell only includes the percentages of atoms contained within the box. Atoms in the unit cell’s corners count as 18 of an atom, atoms on the face count as 12, and an atom in the center counts as a complete atom. Let’s use this to figure out how many atoms are in a simple cubic unit cell, a face centered cubic (fcc) unit cell, and a body centered cubic (bcc) unit cell.
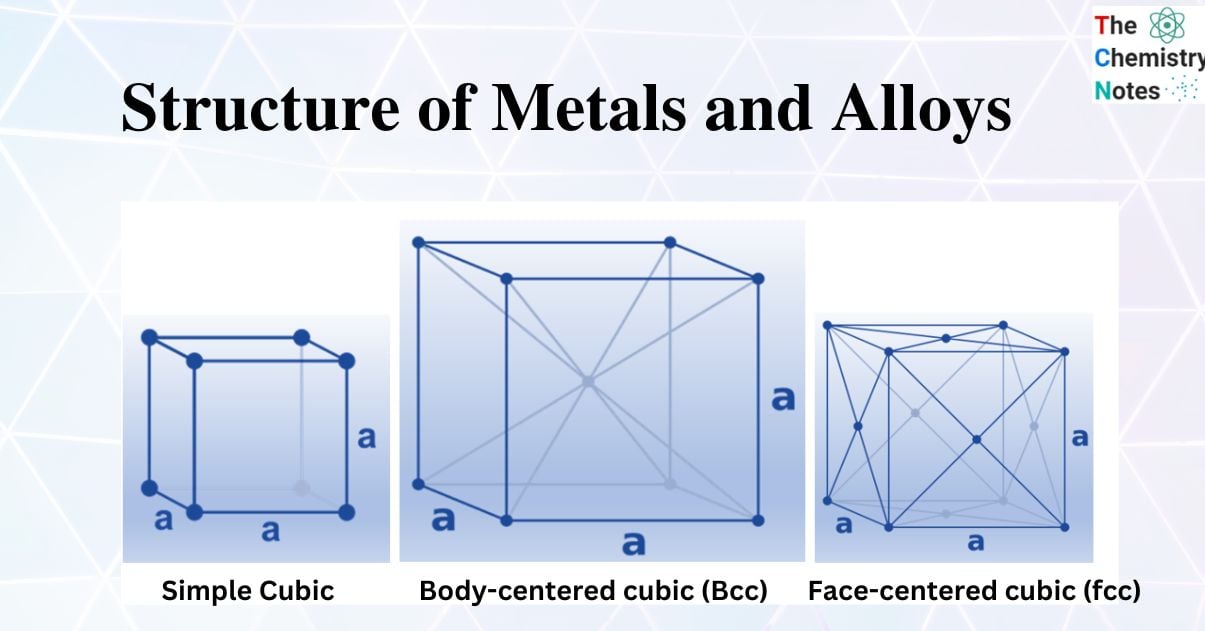
Simple Cubic
The name of this structure is simple cubic packing. In this construction, every sphere touches four other spheres that are identical to it. Additionally, it makes contact with spheres in the planes above and below, respectively. In this arrangement, each atom can create bonds with its six closest neighbors. Thus, it is claimed that each sphere has a coordination number of 6. It is inefficient to use space in a straightforward cubic form. In a straightforward cubic construction, the spheres actually occupy just 52% of the available area. The remaining area is empty. Only one element crystallizes in an easy cubic shape, polonium, due to the inefficiency of this structure.
8 corner atoms × ⅛ = 1 atom/cell. The packing in this structure is not efficient (52%) and so this structural type is extremely rare in metals.
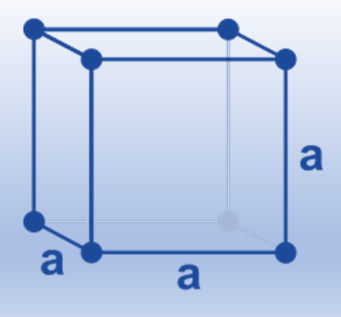
Body-centered cubic (Bcc)
Because each sphere touches four spheres in the plane above and four more in the plane below, positioned toward the corners of a cube, this arrangement is known as a body-centered cubic structure. The body-centered cube is the repeating unit in this construction and is made up of an eight-sphere cube with a ninth identical sphere in the middle. This structure’s coordination number is 8. Body-centered cubic packing utilizes space more effectively than simple cubic packing; in this construction, 68% of the available space is used. A number of the early transition metals, including Ti, V, Cr, Mo, W, and Fe, pack in a body-centered cubic form, as do all of the metals in Group IA (Li, Na, K, and so forth), the heavier metals in Group IIA (Ca, Sr, and Ba), and the metals in Group IIA.
(8 corner atoms × ⅛) + (1 center atom × 1)= 2 atoms/cell. The structure is common for alkali metals and early transition metals, and the packing is more efficient (68%). These structures are also used by alloys such as brass (CuZn).
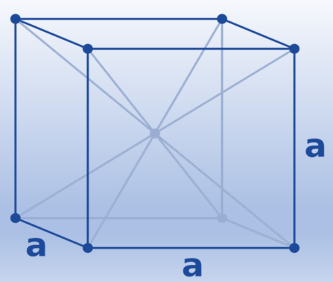
Face-centered cubic (fcc)/ Cubic-closed packed (ccp)
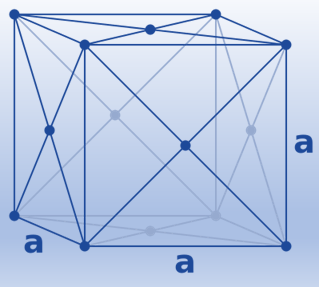
In the cubic closest-packed structure, the atoms in these planes are oriented in different directions. Both the hexagonal and the cubic structures are equally effective when packed closely together. (Both take up 74% of the space.) Many metals crystallize in a cubic closest-packed form, including Ag, Al, Au, Ca, Co, Cu, Ni, Pb, and Pt. When these gases are cooled to low enough temperatures to solidify, they all do as well, with the exception of helium.
The simplest repeating unit in a cubic closest-packed structure is the face-centered cubic unit cell. In fact, the structure’s name, cubic closest-packed, derives from the fact that it contains face-centered cubic unit cells.
(8 corner atoms × ⅛)+ (6 face atoms × ½)= 4 atoms/cell. This structure has the highest efficient packing (74%), along with its hexagonal relative (hcp). Many metals have either a fcc or a hcp structure.
Hexagonal closed packed (Hcp)
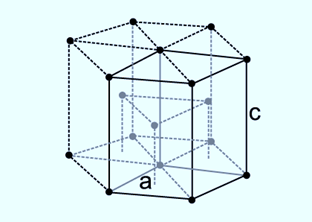
It is known as a hexagonal closest-packed structure because it is made up of spheres that are alternately packed in hexagons on two planes. Each sphere makes contact with six spheres in the same plane, three spheres in the plane below, and three spheres above. In a hexagonal closest-packed arrangement, 74% of the available space is occupied, therefore making the coordination number 12. The hexagonal closest-packed structure is crucial for low-temperature materials like Be, Co, Mg, and Zn as well as the rare gas He because there is no known more effective way to pack spheres. Hence, the packing density of the hcp is 74%, just like the fcc structure.
Grain Structure of Metals and Alloys
Individual crystal clusters, or “grains,” make up the interior structure of a metal. These grains’ shape, size, and orientation are a function of the alloy’s composition and manufacturing process (e.g. forging, casting or additive manufacturing). When the molten material solidifies, grains are created. These grains interact with one another as well as with phases and contaminants. The grain structure is typically adjusted for the technological use. Chiefly, the mechanical and technical qualities of these materials are closely related to the size, orientation, and other structural features of the grains. Additionally, future outside stimuli affect structural properties. These factors comprise:
- Chemical factors (e.g. corrosion)
- Physical or chemical effects (e.g. heat treatment processes)
- Mechanical factors (e.g. following the forming processes, such as forging, rolling, bending, etc.)
When the liquid material solidifies, the grains themselves are produced. Generally, for the use of the metal alloy, the grain structure is modified. Cupro-nickel, for instance, has a grain structure that allows the metal to be crushed into nickels and dimes.
You may determine a material’s characteristics, such as its strength, hardness, and ductility, by looking at the grain structure, also known as the microstructure.
Coordination Number and Structure of Metals
The total number of atoms, ions, or molecules linked to a certain atom is indicated by the atom’s coordination number in a given molecule or crystal. An other name for an atom’s coordination number is “ligancy.”
Ligands are the atoms, ions, or molecules that are linked to the main atom (or molecule/ion). When determining the coordination number of a central atom in a crystal, the calculation of the ligancy of molecules differs.
It is simple to see why metals form structures that are tightly packed in hexagonal or cubic shapes. These structures have the highest possible coordination numbers, which enables each metal atom to establish bonds to the greatest number of nearby metal atoms, in addition to making the most use of available space.
| Structure | Coordination Number | Stacking Pattern |
| Simple Cubic Structure | 6 | AAAAAAAA. . . |
| Body-centered Cubic Structure | 8 | ABABABAB. . . |
| Face-centered Cubic/ Cubic-closest | 12 | ABCABCABC. . . |
| Hexagonal Closed Packing | 12 | ABABABAB. . . |
References
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Book%3A_Introduction_to_Inorganic_Chemistry_(Wikibook)/06%3A_Metals_and_Alloys-_Structure_Bonding_Electronic_and_Magnetic_Properties
- https://www.struers.com/en/Knowledge/Materials/Metallic-grain-structures#main
- https://pocketdentistry.com/1-4-structure-of-metals-and-alloys/
- https://www.studysmarter.co.uk/explanations/chemistry/ionic-and-molecular-compounds/structure-of-metals-and-alloys/
- https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch13/structure.php
