Stability constant (also known as a formation constant or binding constant) is an equilibrium constant for complex formation in solution. Transition element ions are hydrated in aqueous solutions. They are complex ions that contain water as a ligand. Different Ligands combine to form complexes with varying stabilities.

Interesting Science Videos
Stability Constant (Kstab)
Ligands in a complex can be partially or completely exchanged for other ligands if the resulting complex is more stable than the original.
The equilibrium constant for the formation of a transition metal ion complex in a solvent from its constituent ions or molecules is the stability constant (Kstab).
Kstab is a measure of a complex’s stability: the higher the Kstab, the more stable it is.
High Kstab – The position of equilibrium is further to the right. The product has become more stable.
Low Kstab – The equilibrium position is further to the left. The reagent has become more stable.
The stability constants (Kstab) of ligands are frequently given on a log10 scale to make comparisons easier. By comparing the Kstab values of both complexes, Kstab can be used to predict whether a ligand substitution reaction will occur.
For example: When we add concentrated aqueous ammonia to an aqueous solution of copper(II) sulfate, the ammonia ligands displace water ligands in a stepwise process.
As we increase the concentration of ammonia, this process continues until four of the water molecules are replaced by ammonia to form a deep blue solution.

This ligand exchange can be thought of as competing equilibria of forward and backward reactions. The equilibrium point is in the direction of the more stable complex. In this case, the complex containing ammonia is more stable than the complex containing only water. When the complex is diluted with water, the position of equilibrium shifts to the left, forming a complex with more water molecules as ligands.
Equilibrium Expressions for Stability Constants
The complex’s stability is expressed in terms of the equilibrium constants for ligand displacement. This is referred to as the stability constant. Rather than stepwise constants, an overall stability constant, Kstab, is usually given. The stability constant is the equilibrium constant for the formation of a complex ion from its constituent ions or molecules in a solvent. So for the following equilibrium, stability constant is expressed as;

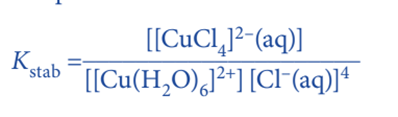
Note: Water does not appear in the equilibrium expression because it is so abundant that its concentration is assumed to be constant.
Units of Stability Constant
The units for the stability constant of above expression is calculated as;
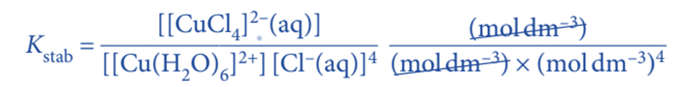
Hence, the unit is dm12mol–4
Stability constants are frequently expressed on a log10 scale. They have no units when expressed on a log10 scale. The stability constants of any two ligands can be used to compare their stability. The values given are typically the complex’s stability relative to the aqueous ion when the ligand is water. The more stable the complex, the higher the value of the stability constant.
Some stability constant values for various copper(II) complexes in relation to their aqueous ions.
| Ligand | log10 Kstab |
| Chloride, Cl– | 5.6 |
| Ammonia, NH3 | 13.1 |
| 2-hydroxybenzoate | 16.9 |
| 1,2-dihydroxybenzene | 25.0 |
Effect of Ligands in Stability Constant
Complexes containing bidentate ions (2-hydroxybenzoate and 1,2-dihydroxybenzene) have higher stability constants in general those containing monodentate ligands The values of the stability constants can be used to predict the effect of adding different ligands to complex ions. Because the stability constant of the ammonia complex is higher than that of the chloride complex, adding excess ammonia to the complex [CuCl4 ] 2-(aq) should result in the formation of a dark blue solution of the ammonia complex. The equilibrium position is shifted to the right, in the direction of the more stable complex.
When excess 1,2-dihydroxybenzene is added to the dark blue ammonia complex, it forms a green complex with 1,2-dihydroxybenzene. This is due to the fact that the stability constant of 1,2-dihydroxybenzene is greater than that of ammonia.
Complexes containing multidentate ligands are more stable than those containing only unidentate ligands. This is due to the fact that each multidentate ligand displaces more than one water molecule. This increases the number of species present in the system and, as a result, the entropy.
Factors affecting the stability constants of complexes
Chelate Effect
As the number of chelate rings increases, so does the chelate effect. The complex [Ni(dien)2)]2+, for example, is more stable than the complex [Ni(en)3)]2+; both complexes are octahedral with six nitrogen atoms surrounding the nickel ion, but dien (diethylenetriamine, 1,4,7-triazaheptane) is a tridentate ligand whereas en is bidentate. The number of chelate rings in the ligand is one less than the number of donor atoms. With six donor atoms, EDTA (ethylenediaminetetracetic acid) forms very strong complexes with five chelate rings. Ligands with eight donor atoms, such as DTPA, are used to form complexes with large metal ions, such as lanthanide or actinide ions, which typically form 8- or 9-coordinate complexes. The most stable complexes are produced by chelate rings with 5 and 6 members.
Because of the ring’s small inter-bond angle, 4-membered rings are subject to internal strain. The chelate effect is also reduced in 7- and 8-membered rings because the larger rings are less rigid and thus lose less entropy in formation.
Deprotonation of aliphatic –OH groups

The removal of a proton from an aliphatic -OH group in aqueous solution is difficult due to the large amount of energy required. As a result, ionization of aliphatic -OH groups occurs only in rare cases in aqueous solution. Compounds with the H2N-C-C-OH substructure are an example of such a situation. Compounds with the 2- aminoethanol substructure, for example, can form metal-chelate complexes with the deprotonated form, H2N-C-C- O. The chelate effect provides the additional energy required to break the -OH bond.
The macrocyclic effect
In comparison to the stability of the complex with the corresponding open-chain amine, the stability of the complex of copper(II) with the macrocyclic ligand cyclam (1,4,8,11-tetraazacyclotetradecane) was found to be much greater than expected. This phenomenon was termed “the macrocyclic effect,” and it was also thought to be an entropy effect. Later research, however, suggested that both enthalpy and entropy factors were at work.
The selectivity for metal ions of macrocyclic ligands over open-chain (chelating) ligands is based on the size of the cavity into which the metal ion is inserted when a complex is formed. For example, the crown ether 18-crown-6 forms much stronger complexes with the potassium ion, K+, than with the smaller sodium ion, Na+.
Haemoglobin
Haemoglobin is a protein found in our red blood cells. This is in charge of transporting oxygen from our lungs to all of our cells. At its core, haemoglobin contains the transition metal ion Fe2+. This is responsible for the red coloration of blood cells.
A transition metal complex known as haem is part of the structure of haemoglobin.
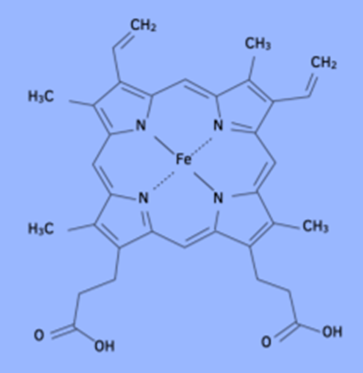
This complex has a co-ordination number of six and an octahedral shape. Four of the ligands, as shown in color, are formed with nitrogen atoms. Below the plane of this structure, there is a fifth nitrogen atom that is part of a protein called globin. The fifth ligand is formed by this. The sixth ligand is composed of oxygen molecules found in high concentrations, such as in the lungs. This results in the complex oxyhaemoglobin. Fe2+ and oxygen molecules have a weak co-ordinate bond.
The co-ordinate bond with oxygen breaks down in cells with low oxygen concentrations, and the oxygen ligand is replaced by a water ligand. The oxygen is delivered to the cells where it is required.
References
- https://www.savemyexams.co.uk/a-level/chemistry/cie/22/revision-notes/6-inorganic-chemistry-a-level-only/6-3-transition-element-complexes-isomers-reactions–stability-a-level-only/6-3-7-effect-of-ligand-exchange-on-stability-constant/
- https://studymind.co.uk/notes/ligand-substitution-reactions/
- https://en.wikipedia.org/wiki/Stability_constants_of_complexes#:~:text=In%20coordination%20chemistry%2C%20a%20stability,together%20to%20form%20the%20complex.
- Beck, M. T.; Nagypál, I. (1990). “Chapter 1”. Chemistry of Complex Equilibria. Horwood. ISBN 0-85312-143-5.
