
Solubility equilibrium is attained when the dissolution and precipitation of a solute species take place at equivalent rates. Solubility equilibrium serves as the foundation for numerous natural and technological processes, spanning from tooth decay to water purification. Understanding the parameters influencing chemical solubility is therefore critical for proper control of these processes.
Interesting Science Videos
Solubility
Solubility is defined as the ability of a solute to dissolve in a solvent and create a solution.
Solubility refers to the capacity of a given solute to dissolve in a particular solvent, ultimately resulting in the formation of a homogeneous solution. This is the characteristic that facilitates the dissolution of substances such as sugar molecules in a cup of coffee. Water is commonly referred to as a “universal solvent” due to its ability to dissolve a wide range of substances, although there exist a limited number of exceptions.
- The aqueous solubility of ionic compounds, which undergo dissociation into cations and anions, exhibits significant variability. Some compounds are highly soluble and may even absorb moisture from the atmosphere, whereas others are highly insoluble.
- The phenomenon of solubility involves the establishment of chemical bonds between the molecules of solute and solvent. The term “solubility” refers to the upper limit of solute concentration that can be dissolved in a given concentration of solvent under specific temperature conditions.
- Solutes can be classified into three distinct categories based on their solubility characteristics, namely highly soluble, sparingly soluble, or insoluble.
- For a substance to exhibit solubility, it must be capable of dissolving at least 0.1 g of solute in 100 mL of solvent. When a solute is dissolved in a solvent at a concentration that is less than 0.1 g, it is commonly referred to as being sparingly soluble.
What is Solubility Product?
The solubility product is a type of equilibrium constant that is temperature-dependent. The solubility product constant (Ksp) typically exhibits a positive correlation with temperature, resulting in an increase in Ksp as temperature rises, owing to the increased solubility.
The term “solubility product” pertains to salts that exhibit low solubility. The maximum value of the product of the molar concentrations of dissociated ions, raised to their respective stoichiometric coefficients, defines the compound’s dissociation constant. The constant solubility product remains unchanged at a specific temperature. A lower solubility product value corresponds to a lower solubility, while a higher solubility product value corresponds to a higher solubility.
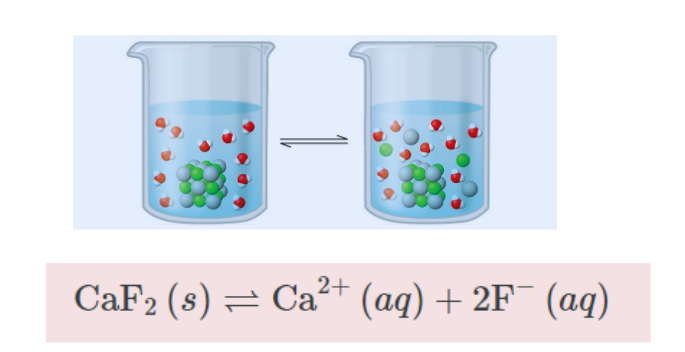
Solubility Product Constant
The solubility product constant represents the equilibrium constant for the dissolution of a solid substance in an aqueous solution. It is represented by the symbol Ksp.
Assume you have barium sulphate and its saturated aqueous solution. The equilibrium between undissolved solids and ions is represented by the following equation:
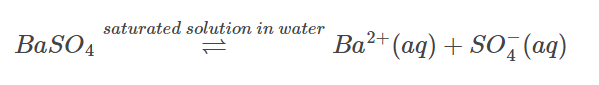
In the prior example, the equilibrium constant is:
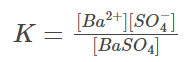
Because the concentration of pure solid substances remains constant, we can say:
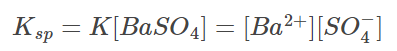
In this case, Ksp stands for the solubility product constant. This also means that when solid barium sulfate is in equilibrium with its saturated solution, the product of barium and sulphate ion concentrations is equal to the solubility product constant.
| Soluble | Solubility > 0.1M |
| Slightly soluble | 0.01M < Solubility < 0.1M |
| Sparingly soluble | Solubility < 0.1M |
Significance of Solubility Product
The solubility product holds great importance in the field of chemistry.
- The solubility of a substance is primarily influenced by the lattice enthalpy of the salt and the solvation enthalpy of the ions present in the solution. The solvation enthalpy of ions is consistently negative, signifying that the process results in the release of energy.
- The determination of solvation enthalpy, which represents the energy released upon solvation, is contingent upon the composition of the solvent.
- Non-polar solvents possess a solvation enthalpy that is relatively low, thereby rendering it inadequate to surmount the lattice enthalpy.
- Consequently, the solubility of the salts is restricted to polar solvents. Thus, for the dissolution of salt in a given solvent to occur, the magnitude of its solvation enthalpy should surpass that of its lattice enthalpy.
- The solubility of a solute can be enhanced by elevating its temperature. At temperatures of 20°C or 100°C, water has the ability to dissolve solutes. The complete liquefaction of soluble solid or liquid substances can be achieved through the elevation of temperature.
- In the context of gaseous substances, it is observed that solubility exhibits an inverse relationship with temperature. Specifically, as the temperature increases, gases tend to expand and consequently escape from their solvent.
Ksp and Solubility
The solubility of a slightly soluble ionic compound can be directly correlated to its Ksp, given that the dissolution process just involves dissociation and solvation.
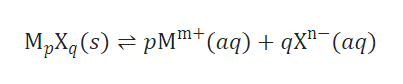
In cases such as these, Ksp values can be derived from solubilities or vice versa. This type of calculation is most easily conducted using a compound’s molar solubility, which is measured as moles of solute dissolved per liter of saturated solution.
Factors Affecting Solubility Product Constant
Temperature: The majority of solutes become soluble as the temperature rises. For instance, coffee dissolves faster in hot water than in frigid water. The solubility of solids and gases, but not liquids, is affected by temperature.
Pressure: The pressure has an impact on the solubility of gases in liquids. The solubility of gases is directly proportional to the partial pressure, according to Henry’s law. If the partial pressure increases, solubility increases and vice versa.
Molecular Size: Smaller solute molecules are more soluble than their larger counterparts. Therefore, smaller molecules can dissolve faster than larger ones.
References
- Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. ISBN 978-0-13-175553-6. Solubilities of ionic salts. Includes a discussion of the thermodynamics of dissolution.
- Stuart, M.; Box, K. (2005). “Chasing Equilibrium: Measuring the Intrinsic Solubility of Weak Acids and Bases”. Analytical Chemistry. 77 (4): 983–990. doi:10.1021/ac048767n. PMID 15858976.
- https://chemistrytalk.org/what-is-solubility/
- Linke, W.F.; Seidell, A. (1965). Solubilities of Inorganic and Metal Organic Compounds (4th ed.). Van Nostrand. ISBN 0-8412-0097-1.
- Kenneth Denbigh, The Principles of Chemical Equilibrium, 1957, p. 257
- https://byjus.com/chemistry/solubility-product-constant/
- https://www.jove.com/science-education/11417/solubility-equilibria
- https://www.geeksforgeeks.org/solubility-equilibria/
- https://edtechbooks.org/general_college_chemistry_2/30_solubility_equili
- https://www.chemistrylearner.com/solubility/solubility-product-constant
