A solid is the state of matter. Solids have fixed shapes and sizes. In any compound, intermolecular forces of attraction and thermal force operate in opposite directions. The intermolecular force of attraction tends to keep the particles together while the thermal force tends to keep the particles apart due to kinetic energy.
State of matter like solid, liquid, and gases are the consequences of the extent of these forces. At high temperatures, thermal force predominates over the intermolecular force so the substance exists in the solid state whereas, at low-temperature, intermolecular force outweighs the kinetic energy and particles cling to one other occupying a fixed position. In a solid state, particles cannot escape from their mean position. This is called a solid state. Solid state chemistry is the study of the synthesis, structure, properties and applications of solids.
Properties of solid
1. They have fixed shape size and volume.
2. They are incompressible and inflexible.
3. The particles constituting a solid may be atoms (e.g, in metal), ions (e.g, in NaCl), or molecules (e.r, dry ice). These occupy a fixed position and only oscillate about their mean position.
4. Molecules have a short intermolecular distance. As a result, the force between constituent particles (atoms, molecules, or ions) is extremely strong.
5. Solids have high density.
6. Particles in a solid do not diffuse or diffuse very slowly.
7. Most of the solid melts on heating. But some solids sublime on heating.
Classification of solids
Based on the arrangement of the constituent particles solids are classified into three types. They are as follows:
1. Crystalline solid
2. Amorphous solid or pseudo solid
3. Polycrystalline or microcrystalline solid
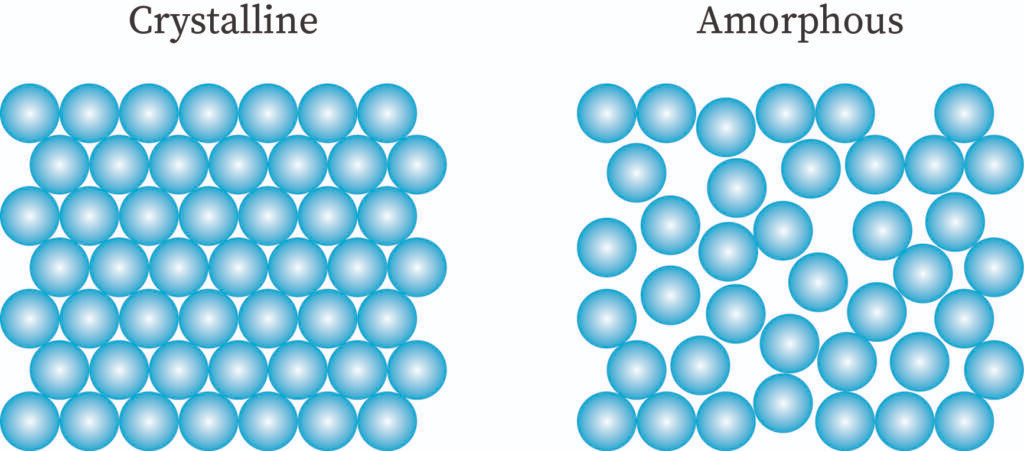
Crystalline solid
A pure, homogenous, solid substance that is composed of atoms, ions or molecules arranged in a regular pattern. It has long-range order which means that there is a regular pattern of arrangement of particles that repeats itself periodically over the entire crystal. Arrangement of minute crystals in well-defined order results in the formation of crystalline solid.
Properties of crystalline solids
- A particular type of crystalline solid is periodically consist of particular constituent units. So crystalline solids have a definite shape.
- Crystalline solids have sharp melting points indicating the presence of long-range order arrangement of a particular constituent.
- When crystalline solids are cut with the edge of a sharp tool they split into two pieces having a plane and smooth surfaces.
- Crystals are bounded by plane faces meeting at a sharp edge.
- Crystalline solids are anisotropic, i.e., their mechanical, electrical, and optical properties depend upon the direction of measurement. This is particularly due to different arrangements of repeated constituent particles of crystal along different directions. For example: in an AgI crystal, the coefficient of thermal expansion is positive in one direction and negative in another direction.
- Crystalline solids have fixed heat of fusion.
Types of crystalline solids
Depending on the nature of bonding the crystalline solids are further classified into four types. They are as follows:
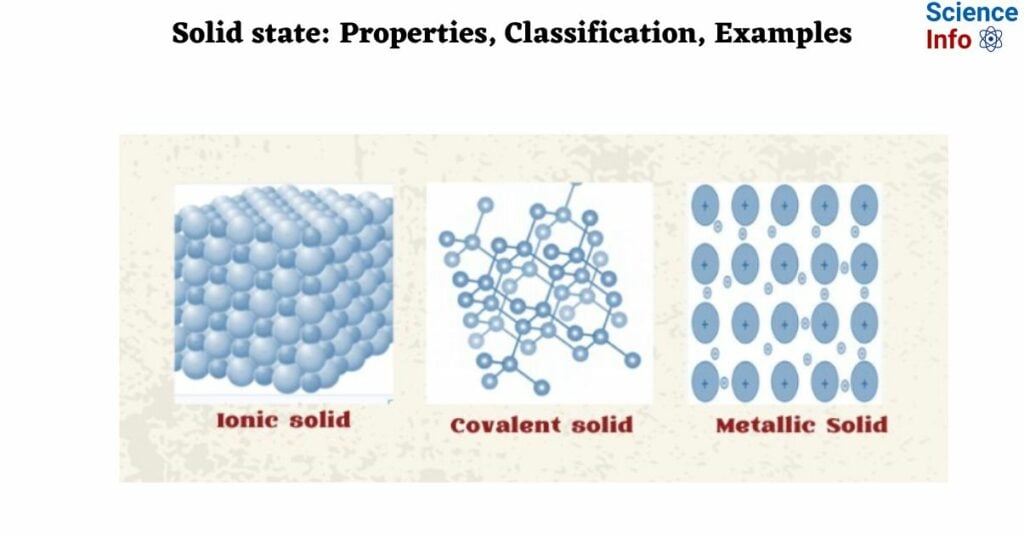
1. Ionic solid
2. Molecular solid
3. Covalent solid
4. Metallic solid
Ionic solid
Ionic solids consist of alternating positive and negative ions. Ions can be either monoatomic or polyatomic.
- In these solids’ ions are held together by a strong electrostatic force of attraction.
- They are hard and brittle.
- They have a high melting point and boiling point.
- They have higher enthalpies of fusion and vaporization than molecular compounds.
- They are good insulators.
- They conduct electricity in solution state.
- Example: NaCl, MgO, ZnS, etc.
Molecular solids
Molecular solids consist of molecules at the crystal’s lattice point that is held together by relatively weak intermolecular forces. The three different types of molecular solids are as follows:
Non-polar molecular solids
These solids consist of molecules or atoms bonded through a non-polar covalent bond. There is a symmetrical distribution of electrons. Atoms or molecules are held together by weak dispersion forces or London forces.
- They are soft in nature.
- They are insulators of electricity.
- They have very low melting points
- Dispersion or London intermolecular forces are present.
- Examples; H2, CO2, CCl4, Cl2, etc.
Polar molecular solids
These solids are held together by polar covalent bonds, while the atoms/molecules are held together by relatively stronger dipole-dipole interactions.
- They are soft in nature.
- Most of them are gases or liquids at room temperature.
- They do not conduct electricity.
- They have high melting point than nonpolar molecular solids.
- Example: HCl, SO2, NH3, etc.
Hydrogen-bonded molecular solids
In these solids, molecules or atoms are held together by a strong hydrogen bond. The properties of hydrogen-bonded molecular solids are as follows:
- At room temperature and pressure, they exist as volatile liquids or soft solids.
- They don’t conduct electricity.
- Their boiling point is low. However, they have a higher boiling point and melting point than polar and non-polar molecular solids.
Example: H2O(ice)
Covalent solids
In these solids, the atoms of the same or different elements are connected by a covalent bond. A network of interconnected covalent bonds formed throughout the crystal results in the formation of a giant molecule.
- Covalent solids are hard. Diamond is the hardest naturally occurring substance.
- They are band conductors of electricity except for graphite.
- They have a high melting point.
- They have high heat of fusion.
- Example: diamond, calcium carbide, graphite, etc.
Metallic solids
Constituent particles of metallic solids are metal atoms. Metal atoms have valence electrons that can be given or lost, resulting in positively charged particles in metallic solids. The available sea of electrons is spread throughout the crystal and can easily move around. So, the formation of metallic bonds is caused by the attractive force between positively charged ions and a sea of electrons.
- They are either soft or hard.
- They are lustrous in nature.
- Metalic solids are malleable and ductile.
- They are good conductors of heat and electricity.
- They have high melting and boiling point.
Amorphous solids or pseudo solids
There is a random arrangement of the constituent particles in the amorphous solids. They are noncrystalline solids with no proper arrangement of atoms in the crystal lattice. They have the properties of rigidity and incompressibility to some extent only. These are not true solid. They lack a distinct geometrical form and have short-range order. Example: plastic rubber etc.
Properties of Amorphous solids
- The position of atoms or molecules is not fixed and varies from one solid to another.
- Amorphous solids have no definite geometry due to the random arrangement of atoms and molecules inside the solid lattice
- They have short-range orders. It means there is presence of a regular and periodic repeating pattern only for short distances.
- As they do not form crystalline structures and can flow, amorphous solids are also known as pseudo solids or supercooled liquids.
- Amorphous solids are isotropic. The physical properties such as refractive index, electrical conductivity, optical density, etc are the same in all directions.
- Because of their irregular packing, amorphous solids do not have a sharp melting point.
- They break into two pieces with irregular shapes by cutting with a sharp edge tool.
- Amorphous solids are unsymmetrical in nature.
- They do not have definite heat of fusion.
Polycrystalline solids
Some substances give an impression of being amorphous but reported to have very fine crystals. Those crystalline substances that resemble amorphous materials in appearance are polycrystalline solids.When crystals form, the neighboring crystals that are next to one interfere with that crystal’s normal growth, which results in the formation of large crystals by packing tiny crystals randomly. This causes the formation of polycrystalline substances. Metals and rocks are polycrystalline substances.
References
- West A. R. (2014). Solid state chemistry and its applications (Second edition student). John Wiley & Sons.
- https://ncert.nic.in/ncerts/l/lech101.pdf
- https://byjus.com/jee/solid-state/
- https://chem.libretexts.org/Courses/College_of_Marin/CHEM_114%3A_Introductory_Chemistry/12%3A_Liquids%2C_Solids%2C_and_Intermolecular_Forces/12.07%3A_Types_of_Crystalline_Solids-_Molecular%2C_Ionic%2C_and_Atomic
- https://www.sjctni.edu/Department/ch/eLecture/Solid%20State.pdf
- https://byjus.com/chemistry/classification-of-crystalline-solids/
- https://www.britannica.com/science/crystal
- https://chem.libretexts.org/Courses/Solano_Community_College/Chem_160/Chapter_09%3A_Liquids_and_Solids/9.5%3A_Molecular_Solids
- https://www.bartleby.com/subject/science/chemistry/concepts/solids
