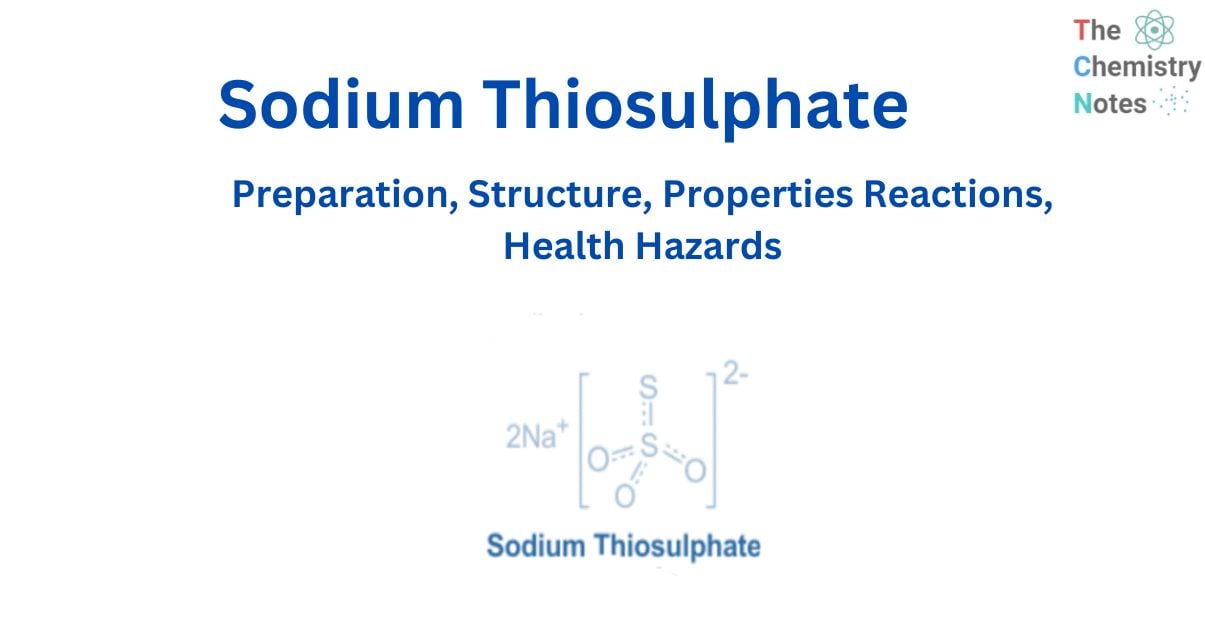
Sodium Thiosulphate is an inorganic salt with the chemical formula Na2S2O3. It is a white colorless crystal or even powder. When degraded into sulfide and sulfate in the air, the material is known to be alkaline. Sodium thiosulphate is soluble in water, producing thiosulfate ions, excellent reducing agents.
When it is decomposed by heat, it generates very poisonous vapors of sulfur oxides. It is frequently discovered in toxic waste from the dye industry. It is an ionic compound composed of two sodium cations and one thiosulfate anion, with one sulfur atom connected to three oxygen atoms and one more sulfur atom. Double bonds connect the oxygen atoms to the sulfur atom, and a single bond connects the other sulfur atom.
It has tetrathionate stability, which means it can form stable tetrathionate complexes in solution. Because of its capacity to effectively neutralize chlorine, sodium thiosulphate is a common component in water treatment dechlorination operations. These properties, combined with their low toxicity, contribute to sodium thiosulphate’s wide range of applications in sectors such as photography, medicine, and analytical chemistry. Historically, sodium thiosulfate has been utilized as a nephroprotectant during the administration of cisplatin and as an antidote for cyanide poisoning. It is believed to have cation-chelating as well as antioxidant effects.
Preparation of Sodium Thiosulphate
- From sodium bisulfite
Sodium thiosulphate and water are formed when sodium hydroxide (NaOH) interacts with sulfur (S) and sodium bisulfite (NaHSO3).
2NaOH + S + 2NaHSO3 → Na2S2O3 + 2H2O
ii. From sodium hyposulphate
It can be prepared by the decomposition of sodium hyposulphate Na2S2O4.
Na2S2O4 → Na2S2O3 + Na2SO3
iii. From SO2
It can be prepared by passing SO2 gas into waste liquor obtained in the manufacture of Na2S. Waste liquor contains Na2S, Na2CO3 and Na2SO3.
2 Na2S + Na2CO3 + 4 SO2 → 3 Na2S2O3 + CO2
2 Na2S + Na2SO3 + 3 SO2 → 3 Na2S2O3
Structure of Sodium Thiosulphate
Sodium thiosulphate is a chemical with a unique structure made up of sodium cations Na+ and thiosulphate anions S2O32-.
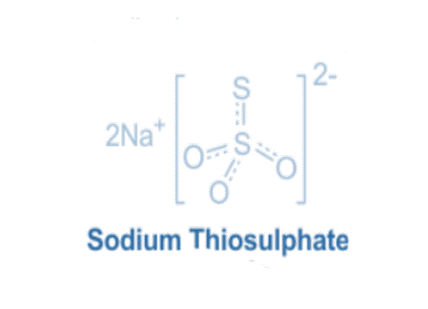
The sodium ion Na+, which has a positive charge, is at the heart of sodium thiosulfate. Sodium is an alkali metal that is well-known for its reactivity and tendency to create cations by losing its outermost electron.
Thiosulphate anions, which contain two sulfur atoms (S) and three oxygen atoms (O), surround the sodium ion. A double bond connects the sulfur atoms, forming a core S-S link. A single connection connects one of the sulfur atoms to an oxygen atom, forming an S-O bond.
The other sulfur atom forms double bonds with two oxygen atoms, resulting in S=O bonds. The thiosulfate anion has a distinct structure that resembles a bent or “U” shape, with the sulfur atoms at the bends and the oxygen atoms projecting outward. Ionic bonding attracts the sodium cations to the negatively charged thiosulfate anions, which keeps the molecule stable.
Physical Properties of Sodium thiosulphate
- Sodium thiosulphate is an inorganic chemical that appears as a white transparent, colorless crystal.
- It is a water-soluble chemical that is also soluble in turpentine oil, but not in alcohol.
- The melting point of the material is between 48 and 52 degrees Celsius.
- This chemical compound is extremely stable in nature and is claimed to be incompatible with certain strong oxidizing agents and acids.
- Thiosulfate anion easily reacts with dilute acids, releasing sulfur, sulfur dioxide, and water.
- The compound has a density of approximately 1.667 g/mL.
Chemical properties of Sodium thiosulphate
- Reducing property
In some chemical processes, sodium thiosulphate acts as a reducing agent. When coupled with an oxidizing agent such as iodine, sodium thiosulfate converts iodine to iodide ions. The following is the reaction:
2 Na2S2O3 + I2→2 NaI+Na2S4O6
- Reaction with salt
A dilute solution of sodium thiosulphate reacts with AgNO3, and a white precipitate of silver thiosulphate (Ag2S2O3) is formed which changes from yellow, orange, brown, and finally to a black precipitate of Ag2S.
2 AgNO3 + Na2S2O3 → Ag2S2O3 + 2 Na2NO3
Ag2S2O3 + H2O → Ag2S+ H2SO4
- Complex formation reaction
Sodium thiosulfate can form complexes with a variety of metal ions. This feature is useful in analytical chemistry, where sodium thiosulphate is widely employed as a titrant to detect the concentration of particular metal ions in solution, such as silver or copper.
6 CuSO4 + 11 Na2S2O3 → 6 Na2SO4 + 3 Na2S4O6 + 2 Na[Cu6(S2O3)5]
- Reaction with acid
When sodium thiosulphate Reacts with acids (HCl, H2S04, HNO3), sulfur dioxide gas is released and Colloidal sulphur (White or yellow turbidity) is formed. The reaction can be expressed as follows:
Na2S2O3 + 2HCl → 2 NaCl + H2O + SO2 + S
Na2S2O3 + H2SO4 → Na2SO4 + H2O + SO2 + S
Na2S2O3 + HNO3 → NaNO3 + H2O + SO2 + S
- Dechlorination reaction
Sodium thiosulphate can be used to dechlorinate water. It interacts with chlorine to produce sodium sulphate and harmless chloride ions, making it very useful in water treatment operations to remove excess chlorine from water supplies.
Na2S2O3 + Cl2 + H2O → Na2SO4 + HCl + S
- Decomposition reaction
Under normal conditions, thiosulphate is a stable chemical. However, when heated, it decomposes to form sodium polysulfide and sodium sulfate.
Uses
- It is essential in the development and fixing of photographic films.
- In the medical field, sodium thiosulphate is extremely important. It is used as an antidote for cyanide poisoning, successfully neutralizing cyanide’s poisonous effects by interacting with it to generate a less hazardous molecule. Because of its feature, sodium thiosulfate is a vital component in emergency medical kits and a lifeline in dire conditions.
- It is used for the determination of the strength of iodine or the determination of the strength of oxidizing agents like potassium permanganate and potassium dichromate.
- Another application for sodium thiosulphate is in the treatment of water. It works as a dechlorinating agent, eliminating chlorine from water. This is especially crucial in aquariums because chlorine can be toxic to aquatic life.
- When dissolved in a large amount of warm water, the chemical can be used as a cleaning agent.
- It is used in the textile industry as a bleaching chemical to help remove undesired dyes and stains from materials. Furthermore, in certain chemical reactions, this molecule works as a chlorine scavenger, preventing the degradation of other compounds due to the presence of chlorine. In industrial environments, where chemical stability is critical, such protective characteristics are highly valued.
Health hazards and precaution
- Direct contact with sodium thiosulphate may cause skin and eye irritation. To avoid any potential contact, it is recommended to wear protective gloves and safety eyewear when handling the compound.
- Inhaling sodium thiosulphate dust or fumes might cause respiratory discomfort. When handling significant amounts of material or generating dust, it is best to operate in a well-ventilated location or wear suitable respiratory protection.
- Some people may experience sensitization or allergic reactions to sodium thiosulphate such as skin rashes, itching, or respiratory symptoms.
References
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/sodium-thiosulfate.
- https://byjus.com/jee/sodium-thiosulphate/
- https://www.vedantu.com/jee-main/chemistry-sodium-thiosulphate
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4750847/
