The size-exclusion chromatography (SEC) method, also known as gel-filtration chromatography, separates biological molecules based on their molecular size. It is not an adsorption technique in comparison to other chromatographic techniques. Size exclusion chromatography is a technique that separates compounds based on differences in molecular size. This process was previously referred to as “gel-filtration” and “gel-permeation chromatography” when the stationary phase is a swollen gel.
SEC is a popular method for purifying and analyzing synthetic and biological polymers such as proteins, polysaccharides, and nucleic acids.
What is Size Exclusion Chromatography?
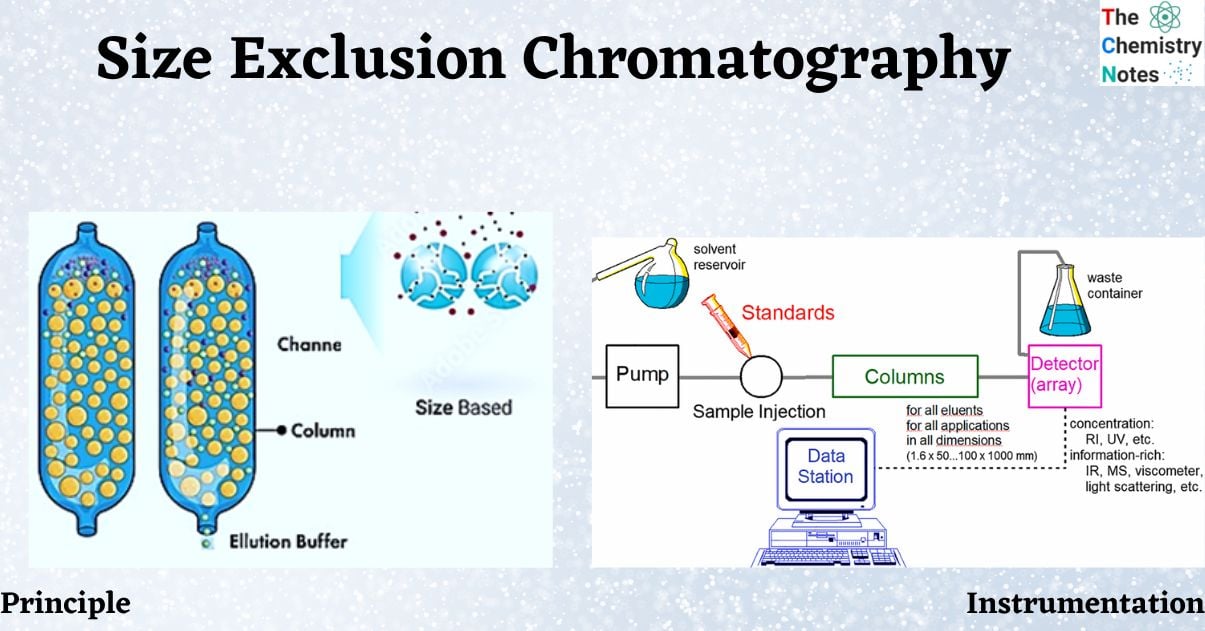
Size exclusion chromatography is a technique for separating different compounds based on their size (hydrodynamic volume), which is determined by how well they penetrate the pores of the stationary phase.
SEC is a popular polymer characterization method due to its ability to provide accurate molar mass distribution (Mw) results for polymers.
Size exclusion chromatography was pioneered by Grant Henry Lathe and Colin R Ruthven, who used starch gels as the matrix to separate analytes of different sizes. Later, Jerker Porath and Per Flodin introduced dextran gels.
There are two basic types of size exclusion chromatography. Gel permeation chromatography (GPC) is used when organic solvents are used. GPC’s primary application field is polymer analysis. Gel filtration is the term used when size exclusion chromatography is performed with aqueous solvents. Protein desalting is a common application of gel filtration.
Size Exclusion Chromatography Principle
The main principle involved in the SEC is solute and solvent molecules diffusion through the pores present in the stationary phase and then removal with the continuous flow of mobile phase.
- Packaging for size exclusion chromatography is typically made of cross-linked polymers such as dextrans, polyacrylamides, styrene, or silica.
- The swelling caused by solvent absorption opens the structure. The size of the holes is determined by the degree of cross-linking. Solute and solvent molecules can diffuse through this network of uniform pores.
- Molecules are effectively trapped and removed from the flow of the mobile phase while in the pores. The effective size of the solute molecules determines their average residence time.
- Larger molecules than the average pore size is excluded. They consequently experience no retention and move through the column at the rate of the mobile phase. Molecules that are significantly smaller than the pore size can penetrate the pore network and thus remain entrapped for the longest period. As a result, the molecules that can penetrate the gel will spend some of their time protected from the moving phase.
- Between these two extremes are intermediate-size molecules, the average penetration of which into packing pores is determined by their diameters. Within this group, fractionation is directly related to molecular size and, to a lesser extent, molecular shape.
It is critical to understand that size exclusions differ from other chromatographic procedures. The analyte and the stationary phase have no physical or chemical interactions in this case. Indeed, efforts are made to avoid such interactions because they may result in decreased column efficiencies.
The following relation can be used to calculate the retention volume VR.
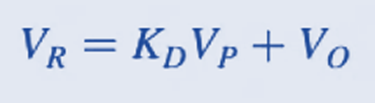
where KD = distribution coefficient, i.e. available pore fraction
VP = the pore volume of the packing medium.
Vo= void volume
Instrumentation of Size Exclusion Chromatography
The bare minimum specifications are an SEC instrument with a solvent delivery pump (with high flow accuracy), a high-pressure injection system (manual or automated), one or more SEC column(s), one or more detector(s), and a computer with software that allows acquiring, calibrating, and analyzing data. To manage eluents safely, a solvent reservoir, waste bottle, and solvent retainer (for the glass bottles) are thus needed.
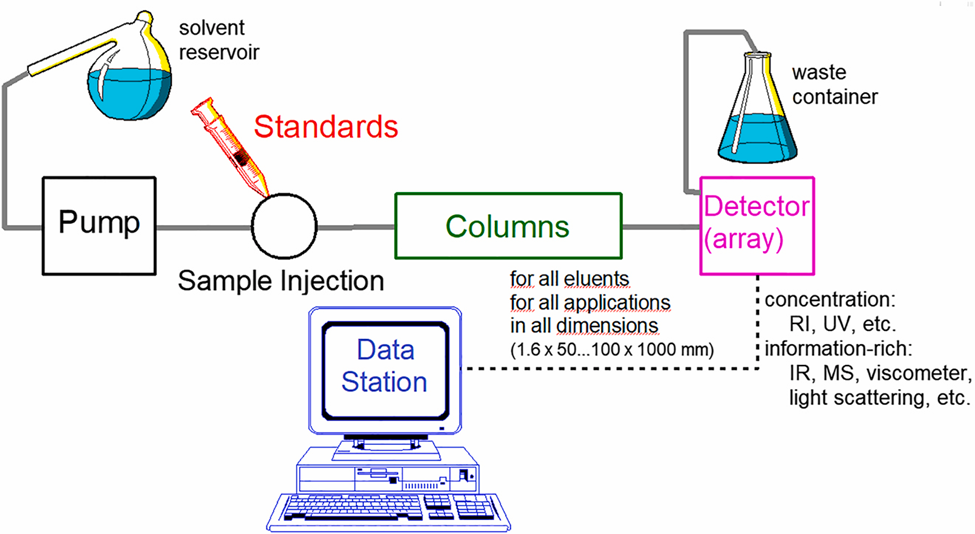
Image Source: [https://doi.org/10.1515/cti-2020-0024]
Mobile phase reservoir
Reservoirs are frequently made of glass or stainless steel. The only precaution that should be taken is that the degasser must first degas the system in order to remove any dissolved gases.
Pumps
These are primarily used to continuously pump the mobile phase at a constant pressure of 6,000 psi through the injector. The flow rate fluctuates between 1 and 10 ml per minute. In the SEC, two different types of pumps are frequently used.
Syringe pumps: These devices function like regular syringes and deliver the mobile phase through a pulse-free flow.
Reciprocating pumps: Single-piston reciprocating pumps are inexpensive but are unsuitable for the SEC. The dual piston pumps are widely used in the SEC because they are simple to maintain.
Injector
This is used exclusively to inject the solvent into the column. The solvent dissolves the sample. Small mobile volumes, between 1 and 500 μl, are required.
Column
Smooth bore glass or steel tubing with a thick wall is used as a column. Column temperature is kept at 105 °C by thermostats, and it is cooled by enclosing it in a water-filled cooling jacket. Styrene and divinyl benzene are just a couple of the organic gels or porous silica that fill the column.
Detectors
The detectors are mainly used to detect the sample constituents and concentration of the sample.
Recorders
Recorders are frequently used for data analysis and recording after a detector has collected data.
Stationary Phase and Mobile Phase Gels
Stationary Phase: Cross-linked dextran, agarose, polyacrylamide, poly acryloyl morphine, and polystyrene are some of the commonly used stationary phase gels. These beads have a clearly defined range of pore sizes and are semi-permeable. For the quick desalting or purification of proteins, small pores sizes are used. To separate relatively small proteins, intermediate pore sizes are employed. Biological complexes are purified using very large pores.
There are three varieties of gel:
Soft gel: Separation of proteins including polyacrylamide gel and dextran (Sephadex).
Semi-rigid gels: Separation of non-polar polymers in non-polar solvents, such as bio beats
Highly rigid gels and glasses: Separation of polar solvents.
To avoid breaking or bursting of the column due to the gel’s swelling, these gels or stationary phases are soaked overnight prior to column packing.
Mobile Phase
In most cases, a buffer is the substance that dissolves the biomolecules to create the mobile phase. The mixture of biomolecules that have dissolved in the buffer is then referred to as the sample. The type of separation to be accomplished and the components to be separated will determine the mobile phase to be used in any separation. The sample must be completely dissolved by the solvent.
For Example: dimethylformamide, chloroform, and tetrahydrofuran.
Factors Affecting Resolution in Size Exclusion Chromatography
Dimensions of the Columns
Increased column length results in increased resolution, while increased column diameter results in increased bed volume and thus higher column capacity. Varying the pore size can control the fractionation range and the exclusion limit. The higher the resolution, thus results in the smaller the particle size of the gel.
The flow rate
For the best resolution, this should be moderate. This is because moderate flow rates allow molecules to use the entire surface area of the gel beads in the stationary phase, allowing smaller molecules to take their time entering the pores. This leads to better separation of molecules of various sizes. Slow flow-rates cause peak over diffusion, thus resulting lower resolution.
Packing of Columns
Overpacking and underpacking can both reduce the degree of resolution. The presence of dead volume on top of the column also has a significant impact on resolution because the molecules in the sample diffuse before entering the column bed, resulting in broad bands or wider peaks.
Detection Method
During SEC analysis, UV is the most commonly used mode of protein detection and measurement. Aromatic amino acids, such as tryptophan, are more sensitive to near UV or longer wavelengths. However, higher wavelengths have a wider linear dynamic range, while lower wavelengths have higher sensitivity and can analyze proteins in low concentrations.
Applications of Size Exclusion Chromatography
The use of size exclusion chromatography in the separation or purification of sugars, polypeptides, viruses, proteins, enzymes, hormones, antibodies, nucleic acids (DNA and RNA), and other substances has many advantages.
- Proteins and other water-soluble polymers are the primary targets of SEC for separation and analysis. It is also used to assess the stability and characteristics of naturally occurring organic matter found in water.
- Size exclusion chromatography for the purification of proteins: The separation of one or more proteins from a complex mixture is accomplished through a series of chromatographic procedures.
- One such method of size-exclusion used to separate proteins according to their sizes is gel filtration or gel permeation chromatography. According to their molecular weight, it is used to analyze molecules. In the biopharmaceutical sector, size exclusion chromatography is thus used to track protein aggregation.
- Molecular weight determination: Analyzing a mixture of molecules with various molecular weights is another use for the SEC procedure. By carefully choosing the gel type and column height, it is also used to separate molecules with the same molecular weight.
- Deselecting: Another use of the SEC procedure is deselecting. The removal of inorganic and ionizable species from biological macromolecules is what it entails. For instance, a Sephadex gel column can separate hemoglobin from sodium chloride.
- Biopharmaceuticals are sophisticated pharmaceuticals made from living organisms or cells. They also can be created using a variety of biotechnological techniques.
- The development and purification of such biopharmaceuticals heavily utilizes the size exclusion chromatography principle or procedure.
Advantages and Disadvantages
Advantages
- Reliable method that works well for handling biomolecules that are delicate to pH changes, metal ion concentrations or co-factor concentrations, or harsh environmental conditions.
- The conditions can be changed without affecting the separation to suit the type of sample or the needs for additional purification, analysis, or storage.
- Short and clearly defined separation.
- Depending on the needs of the experiment, separations can be carried out in the presence of necessary ions or cofactors, detergents, urea, and guanidine hydrochloride, at high or low ionic strengths, at 37 °C, or in the cold room.
- Sensitivity is high in narrow bands.
- Selectivity is high.
- High definition because molecules do not bind to the chromatography medium, unlike in ion exchange or affinity chromatography, buffer composition does not directly influence resolution
- Separation is relatively simple.
Disadvantages
- The chromatogram has a small scale and limits the number of peaks that can be resolved.
- Inapplicability to similar-sized samples
- The analysis requires the use of standards.
- Most of the chains’ molecular masses will be too close for the separation to show anything more than broad peaks.
- High price.
- Dust and solvent concentration are not detected as interfering factors.
The main limitation of the Size Exclusion Chromatography method is that it is difficult to obtain a pure size-exclusion separation for various factors such as concentration effects, osmotic effects, side processes, secondary retention mechanisms, secondary exclusion, SEC band broadening, parasitic processes, and preferential interactions.
References
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3556795/
- http://www.sciencedirect.com/science/article/pii/S0731708514002027
- Sun T, Chance RR, Graessley WW, Lohse DJ (2004). “A Study of the Separation Principle in Size Exclusion Chromatography”. Macromolecules. 37 (11): 4304–4312. Bibcode:2004MaMol..37.4304S. doi:10.1021/ma030586k. ISSN 0024-9297.
- https://www.bio-rad.com/en-np/applications-technologies/introduction-size-exclusion-chromatography
- Pharmaceutical and Biomedical Applications of Polymers, Basic Fundamentals of Drug Delivery https://doi.org/10.1016/B978-0-12-817909-3.00006-6
- Isolation/Purification of Proteins, Encyclopedia of Cell Biology https://doi.org/10.1016/B978-0-12-394447-4.10018-5
