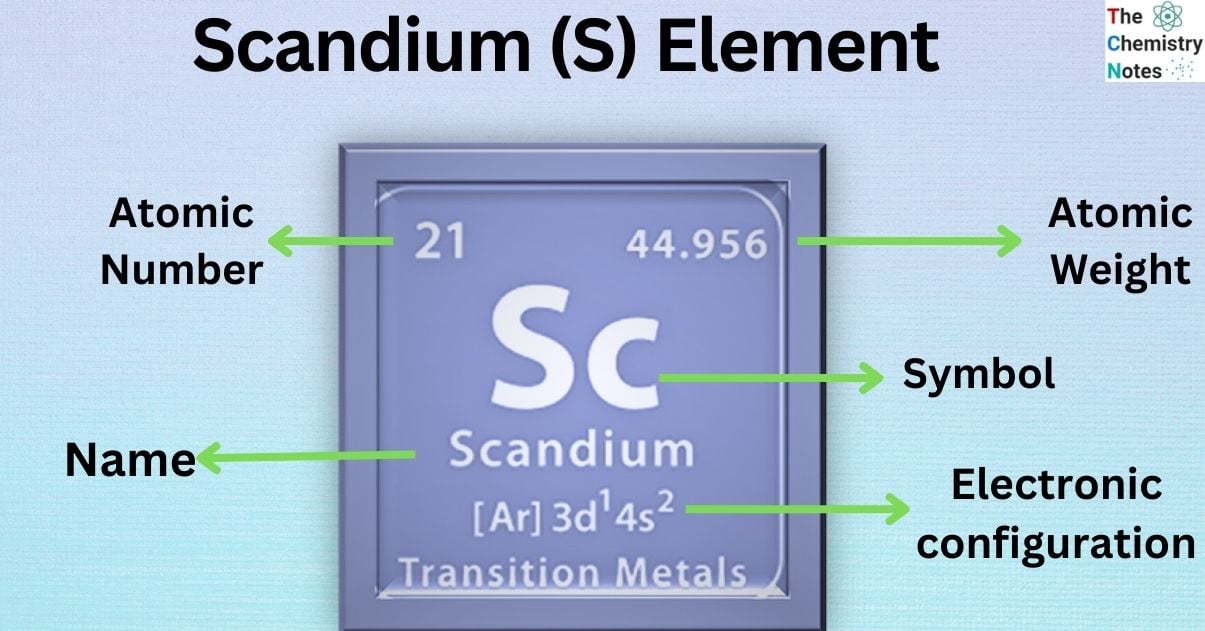
Scandium is the chemical element that belongs to the group 3 of the periodic table. It is classified as the transition and rare earth metal which is represented by the symbol ‘Sc’ and has the atomic number 21 in the periodic table. The name comes from the Latin word ‘Scandia,’ meaning Scandinavia.
Scandium is the first transition element, and it sits between between the conventional rare-earth elements and the light metallic elements. ‘Sc’ is extracted from a rare mineral found in Scandinavia and when exposed to air, it gets a yellowish or pinkish hue. Due to its extremely low occurrence rates, scandium is a rare element in nature and is typically found exclusively in two types of ores.
Interesting Science Videos
History of Scandium
Scandium’s presence was anticipated roughly ten years before it was discovered. How amazing was this.
- Lars Fredrik Nilson, a Swedish chemist, and professor at Uppsala University, discovered Scandium in the year 1879.
- Mendeleev predicted the existence of “eka-boron“,which would have an atomic weight between 40 of calcium and 48 of titanium, based on the Periodic System.
- Nilson identified the element in the minerals euxenite and gadolinite in 1878, which had only been found in Scandinavia.
- In the year 1879, Swedish scientist Per Theodor Cleve established that scandium was the “eka-boron” predicted by Mendeleev.
- Scandium was discovered during the attempt to make a pure ytterbia sample from 10 kg of euxenite ((Y, Ca, Er, La, Ce, U, Th) (Nb, Ta, Ti)2O6).
- Fischer, Brunger, and Grienelaus created metallic scandium for the first time in the year 1937.
- Scandium uses did not emerge until the 1970s, when the favorable effects of scandium on aluminum alloys were identified.
Occurrence of Scandium
Scandium is the 50th most abundant element on Earth, and it is widely dispersed, appearing in tiny amounts in over 800 minerals.
It is a major component of the rare mineral thortveitite, which is found in Scandinavia and Malagasy.
- The abundance of Sc in the Earth’s upper continental crust is estimated to be about 14 milligrams per kilogram (mg/kg).
- The majority of scandium is now recovered from thortveitite or removed as a byproduct from uranium mill tailings.
- Only 50 kilograms of Scandium is produced globally each year. There is no suggestion as to how much could possibly be available.
- Of the plants, that were analyzed for Scandium only about 3 percent were found to possess a tiny amount. Of which vegetables contained only 5 ppb although the grass was found with 70 ppb.
- Pure scandium is now produced by reducing scandium fluoride with calcium metal.
Isotopes of Scandium
- Scandium contains 13 isotopes with known half-lives, ranging in mass from 40Sc to 52Sc.
- Natural Scandium (Sc) is composed of only one stable isotope, 45Sc.
- 13 radioisotopes have been identified, with 46Sc having the longest half-life (83.8 days), 47Sc with a half-life of 3.35 days, and 48Sc with a half-life of 43.7 hours.
- All of the remaining radioactive isotopes have half-lives of less than 4 hours, with the bulk having half-lives of less than 2 minutes.
46Sc is a beta emitter that has been employed as a tracer in crude oil crackers (the process of turning unrefined oil into gasoline and other lower-molecular-weight hydrocarbon fractions). Its beta radiation allows it to be traced as the oil moves.
Elemental Properties of Scandium
| Electronic Configuration | [ Ar ] 3d1 4s2 |
| Atomic Number | 21 |
| Atomic Weight | 44.9559 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 3, 4, d-block |
| Density | 3.0 g.cm-3 at 20°C |
| Ionic radius | 0.083 nm (+3) |
| Van der Waals radius | 0.161 nm |
| Electron shells | 2, 8, 9, 2 |
| Electrons | 21 |
| Protons | 21 |
| Neutrons in most abundant isotope | 24 |
Physical Properties of Scandium
- Scandium is a delicate, light silvery-white metal that becomes slightly yellow or pink when exposed to air.
- It is almost as light as aluminum (low density), but has a significantly higher melting point.
- The taste and odor of Scandium are undetermined.
- ‘Sc’ is in a solid state at a room temperature.
- Scandium is malleable and ductile.
- Sc is often regarded as a nonmagnetic element.
- This substance is not only unusual in terms of the element’s valency (3), but it is also an excellent conductor of electricity for reasons that have yet to be discovered.
- Scandium has a density of 2.99 grams per cubic centimeter. It has a melting point of 1541°C and a boiling point of 2836°C.
- Scandium is the most effective known microalloying element for strengthening aluminum while simultaneously improving flexibility, heat and corrosion resistance, and weight.
| Color/physical appearance | Silvery-white metallic |
| Melting point/freezing point | 1541°C, 2806°F, 1814 K |
| Boiling point | 2836°C, 5137°F, 3109 K |
| Density | 3 (g cm−3) |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.36 (Pauling Scale) 1.19 (Allen Scale) |
Chemical Properties of Scandium
- Scandium is similar to rare earth elements chemically.
- If scandium is exposed in air it will develop a layer of an oxide, which appears to be slightly yellow or pinkish in color.
- It weathers easily and dissolves slowly in most diluted acids.
- When scandium turnings are ignited in the air, it forms scandium oxide and emits a bright yellow flame.
Chemical Reaction of Scandium
- Reaction of scandium with air
Scandium metal tarnishes in the air and easily burns to generate scandium (III) oxide, Sc2O3.
4Sc + 3O2 → 2Sc2O3
- Reaction of scandium with water
Scandium metal dissolves in water when finely split or heated, forming solutions containing the aquated Sc(III) ion and hydrogen gas, H2.
2Sc(s) + 6H2O(aq) → 2Sc3+(aq) + 6OH–(aq) + 3H2(g)
- Reaction of scandium with the halogens
Scandium reacts strongly with the halogens fluorine (F2), chlorine (Cl2), bromine (Br2) and iodine (I2) forming the trihalides scandium(III) fluoride (ScF3), scandium(III) chloride (ScCl3), scandium(III) bromide (ScBr3), and scandium(III) iodide (ScI3) respectively.
2Sc(s) + 3F2(g) → 2ScF3(s)
2Sc(s) + 3Cl2(g) → 2ScCl3(s)
2Sc(s) + 3Br2(g) → 2ScBr3(s)
2Sc(s) + 3I2(g) → 2ScI3(s)
- Reaction of scandium with acids
Scandium metal dissolves readily in dilute hydrochloric acid to form solutions containing the aquated Sc(III) ion together with hydrogen gas, H2.
2Sc(s) + 6HCl(aq) → 2Sc3+(aq) + 6Cl–(aq) + 3H2(g)
Uses of Scandium
- Aluminum and scandium alloys are utilized in sporting equipment such as baseball bats, bicycle frames, and lacrosse sticks. Scandium-aluminum alloys are projected to play a key role in the production of fuel cells.
- Scandium-aluminium alloys were first used as nose cones for Soviet submarine-launched ballistic missiles because they permitted the missile to break through the Arctic ice cap (from underneath) without being damaged.
- Scandium is mostly utilized in research. It has considerable promise, though, because it has virtually the same density as aluminum and a significantly higher melting point. In Russian MIG fighter jets, an aluminum-scandium alloy was utilized.
- Scandium alloys are also utilized in high-end lighting. The presence of scandium provides light that resembles natural sunshine.
- Scandium iodide is added to mercury vapour lamps to produce a highly efficient light source resembling sunlight. These lamps help television cameras to reproduce colour well when filming indoors or at night-time.
- Scandium-46, a radioactive isotope, is employed as a tracer in oil refining to track the movement of different fractions. It may also be used to identify leaks in subsurface pipelines.
- Each year, around 80 kg of scandium is utilized in metal-halide lamps/light bulbs across the world.
- Scandium has significant promise for usage in the aerospace sector and in renewable energy resources, but limited production volumes and a lack of knowledge of scandium in geological systems limit its use globally.
- The Museum’s study will help us better comprehend scandium in the solid-Earth system and evaluate its mineral residency in the geological realm. It may also indicate secondary or main ore deposit recovery prospects.
Health Effects of Scandium
Scandium in its elemental form is considered to be non-toxic. Scandium (Sc) has several industrial applications, but little is known regarding its physiological and/or toxicological consequences.
But some research has shown the effect of scandium compounds on rats. so it would be better to handle the compounds of scandium as compounds with moderate toxicity. The body seems to be handling scandium in a similar manner to gallium, with similar hazards.
- Scandium is mostly hazardous in the workplace because damps and fumes can be inhaled with air. This can result in pulmonary embolisms, especially if exposed for an extended period of time. Scandium, when accumulated in the human body, can be harmful to the liver.
- Scandium is not toxic, although there have been suggestions that some of its compounds might be cancerogenic.
Environmental Effects of Scandium
- Scandium is discharged in the environment in a variety of ways, mostly by the petroleum industry. It can also enter the environment when home items are discarded. Scandium will progressively build in soils and water, leading to increasing concentration in humans, animals, and soil particles.
- Scandium induces cell membrane damage in aquatic animals, which has a number of severe effects on reproductive and nervous system activities.
Biological Importance of Scandium
Scandium serves no biological use. Because only trace amounts make their way into the food chain, the typical person’s daily consumption is less than 0.1 microgram.
Watch out this video for more interesting information about Scandium.
References
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing.
- Samson, Iain M.; Chassé, Mathieu (2016), “Scandium”, in White, William M. (ed.), Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth, Cham: Springer International Publishing, pp. 1–5, doi:10.1007/978-3-319-39193-9_281-1, ISBN 978-3-319-39193-9
- Holleman, A. F.; Wiberg, E. “Inorganic Chemistry” Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- https://www.webelements.com/scandium/
- https://www.rsc.org/periodic-table/element/21/scandium
- https://www.britannica.com/science/scandium
- https://www.chemicool.com/elements/scandium.html
- Mary Elvira Weeks, Discovery of the Elements., Kessinger Publishing, 2003, p679, 680.
- https://pubchem.ncbi.nlm.nih.gov/element/Scandium

