Salicylaldehyde is a chemical compound with the formula C7H6O2. It is also known as 2-Hydroxybenzaldehyde. It is one of three hydroxybenzaldehyde isomers. It is a frequent reactant in the synthesis of numerous organic compounds, including cyclic organic compounds like coumarin and organic ligands like salan ligand.
It is employed in the synthesis of coumarin, saligenin, and salicylaldoxime (an important analytical reagent), as well as in analytical chemistry, such as the detection of hydrazine. Furthermore, salicylaldehyde is a significant precursor of a variety of chelating agents as well as a flavoring ingredient.
It’s a clear or pale yellow liquid with a bitter almond odor and a burning flavor. It is somewhat soluble in water and soluble in alcohol, benzene, and ether. Salicylaldehyde is found in shrubs of the genus Spiraea and is usually generated from phenol by the action of chloroform in the presence of an alkali base.
Interesting Science Videos
Preparation of Salicylaldehyde
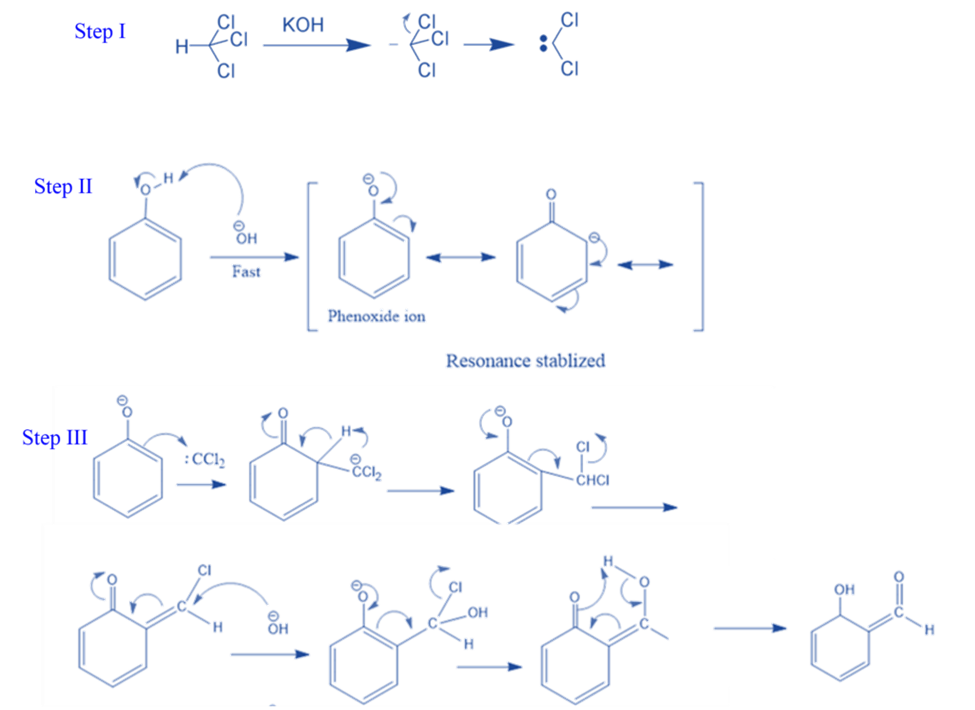
Salicylaldehyde is formed by heating phenol and chloroform in the presence of sodium hydroxide and potassium hydroxide. This is known as the Reimer-Tiemann reaction.
Step I: First, a strongly basic hydroxide solution is used to deprotonate the chloroform. Deprotonation is the elimination of the hydrogen atom. The chloroform carbanion is formed by removing the hydrogen atom. The chloroform carbanion will swiftly undergo alpha elimination, yielding dichlorocarbene(CCL2), the reaction’s major reactive species.
Step II: Base extracts the hydrogen atom from the -OH group to generate the phenoxide ion. The phenoxide’s negative charge is delocalized into the aromatic ring, making it more nucleophilic.
Step III: The addition of dichlorocarbene results in the formation of an intermediate i.e., dichloromethyl-substituted phenol. The resulting intermediate is then exposed to basic hydrolysis to yield ortho-hydroxybenzaldehyde.
Physical properties of Salicylaldehyde
- It is a colorless, clear, oily liquid.
- It has a bitter almond odor.
- It has a melting point of -7° and a boiling point of 196-197°.
- It is slightly soluble in water and soluble in ether.
- It is stable.
- It is combustible.
- It is incompatible with strong bases, strong reducing agents, strong acids, and strong oxidizing agents.
Reactions of Salicylaldehyde
Benzofuran can be prepared by the reaction of salicylaldehyde with alpha halo ketone through intermolecular aldol condensation of intermediate product.

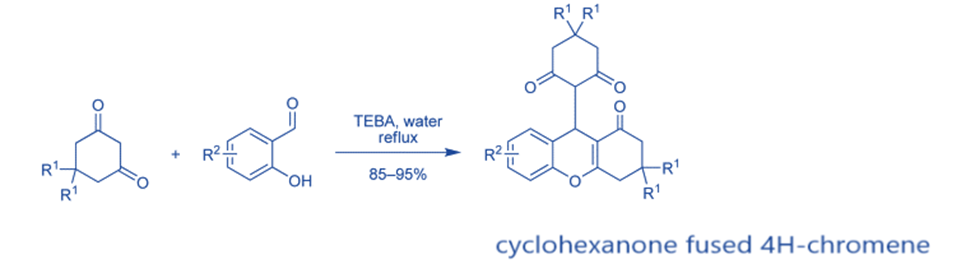
1,3-hexanediones react with salicylaldehydes in the presence of triethylbenzylammonium chloride (TEBA) to produce cyclohexanone fused 4H-chromenes in high yield.
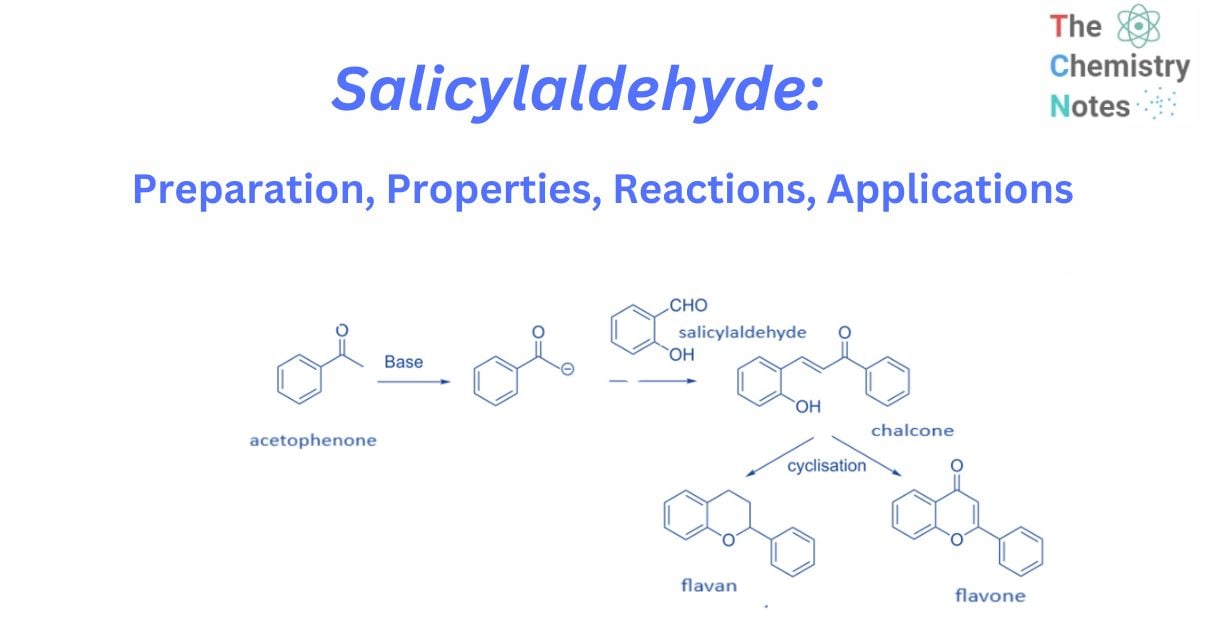
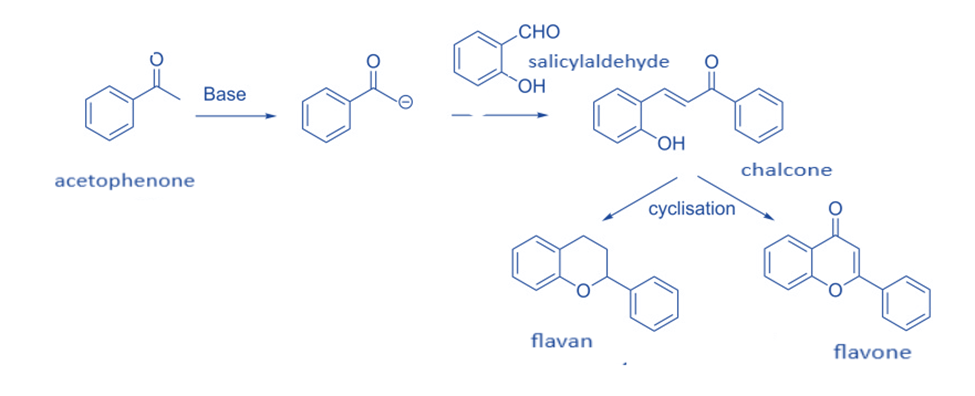
A number of chemists have used the reaction of salicylaldehyde and enolates produced from acetophenone in the synthesis of flavans and flavones.
In general, flavans and flavones are synthesized by treating acetophenone with a base to produce enolate, which is then Knoevenagel condensation with salicylaldehyde to produce chalcone. These chalcone derivatives are subsequently cyclized utilizing a variety of methods to yield flavan or flavone.
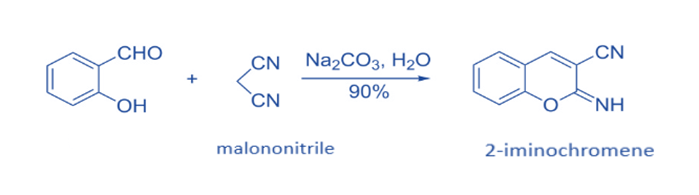
The one-pot reaction of salicylaldehyde with malononitrile has been shown to be an efficient approach for producing 2-iminochromene derivatives. In general, such synthetic methods begin with a Knoevenagel condensation and then progress to intramolecular cyclization.2-iminochromene is formed when salicylaldehyde is reacted with 1 equivalent of malononitrile in the presence of Na2CO3 and H2O as the solvent. When NaHCO3 was utilized as the base instead of Na2CO3, a comparable yield was obtained.
Applications of salicylaldehyde
- It is utilized in the production of various commercial items, including shampoos and food preservatives.
- Salicylaldehyde proved to be the most effective and was used in the creation of a simple and sensitive approach for determining Fe(III) in estuary sediments.
- It can be used in medical treatment to detect ketonuria.
- It also acts as a chelating agent for heavy metal ions such as Copper(II) ions, making it useful for colorimetric detection of heavy metal ion concentrations in water samples.
- It is also a precursor in the total synthesis of coumarin (C9H6O2), a white organic crystal with antimicrobial properties.
- Salicylaldehyde is a flavor and fragrance ingredient.
- Salicylaldehyde is a widely utilized highly functionalized arene that can be employed as a precursor in the synthesis of other compounds.
References
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- Smith M. & March J. (2001). March’s Advanced Organic Chemistry: Reactions Mechanisms and Structure (5th ed.). Wiley.
- Ghosh, S.K., Advanced General Organic Chemistry, Second Edition, New Central Book Agency Pvt. Ltd., Kolkatta, 2007.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992. production of polyesters, polyurethanes, and alkaline resins.
