The metals acquired through any method discussed in Metallurgy, typically those with lower electropositivity, require additional purification also called refining of metals due to the potential presence of other metals, dissolved oxides, carbon, phosphorous, and similar impurities. The process refining of metal plays a pivotal and indispensable role in the field of metallurgy. Typically, the metal that has undergone the process of extraction from its ore tends to possess inherent impurities. This rough metal that is extracted is called “crude metal.”
In order to produce very pure metals, contaminants must be removed by refining. Multiple techniques are used for removing contaminants from crude metal, taking into consideration the unique attributes associated with the metal itself and the impurities. There are several methodologies employed in the purification of crude metal.
Interesting Science Videos
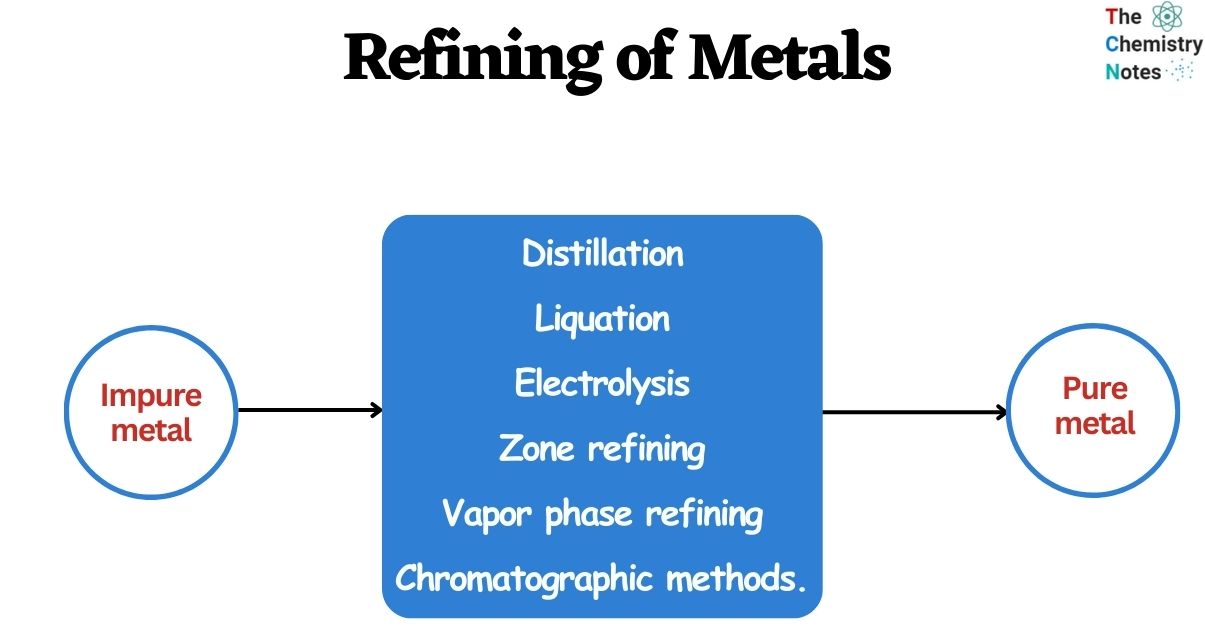
Methods of Purification/Refining of Metals
Different techniques employed for the refining of metals have been briefly discussed here:
Refining By Poling
- Metals, when extracted from their ores, often carry impurities in the form of their own oxides. For instance, blister copper is known to contain Cu2O, while impure tin may contain SnO2, among other examples.
- When molten metal is agitated using green wood poles, it results in the liberation of gases such as CH4 and C2H6. These gases play a crucial role in reducing metal oxides, thereby facilitating the release of free metals.
4 Cu2O + CH4 → 8 Cu + 2 H2O + CO2
(wood pole)
2 SnO2 + CH4 → 2 Sn + 2 H2O + CO2
- The process of refining through reduction is commonly referred to as poling.
Distillation
- This particular technique is utilized for metals that possess a high degree of volatility and exhibit a low boiling point. Metals such as Zinc (Z), mercury, and Cadmium (Cd) exhibit remarkably low boiling points, with zinc boasting a temperature of 407 °C, mercury at 365.58 °C, and cadmium reaching 765 °C.
- The process involves subjecting the impure metal to heat within a retort, resulting in the vaporization of the metal. This vapor is then carefully condensed in a separate receiver, allowing for the isolation and collection of the purified metal.
- The process of distillation causes the pure metal to separate and rise above, while the impurities, which lack volatility, remain trapped within the retort. Zinc, mercury, and cadmium exhibit a pronounced tendency to undergo vaporization when subjected to heat, thereby relinquishing their impurities in the process.
- After that, the leftover material is heated in a furnace to temperatures higher than its melting point. This causes a phase transition where the material becomes gaseous and the impurities are separated from the desired metal.
Electrolytic Refining
- This process involves the utilization of impure metal alongside a strip of metal that is of pure composition. The anode is composed of a metal alloy with impurities, while the cathode is comprised of a singular piece of high-purity metal.
- The objects undergo immersion in an electrolytic solution that consists of a soluble salt composed of identical metals. During the process of electrical conduction within the solution, the unrefined metal undergoes dissolution at the anode, while the refined metal accumulates at the cathode.
- Simultaneously, the impurities remain within the solution contained in the cell or precipitate, resulting in the formation of two distinct substances known as tank mud and anode mud. Copper (Cu), tin, lead, Aluminium (Al), silver, zinc, and other similar metals undergo the refining process in this manner.
Oxidative Refining
- During this process, if any of the impurities in the extracted metals have a tendency to oxidize, they can be removed by vigorously stirring the molten material in the presence of air.
- During the purification of tin, impurities manifest themselves as a layer of scum on the surface, necessitating the implementation of an effective removal technique known as skimming.
S + O2 → SO2
C + O2 → CO2
P + O2 → P2O3 or P2O5
As + O2 → As2O3 or As2O5
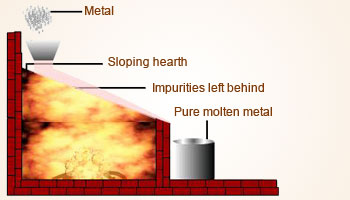
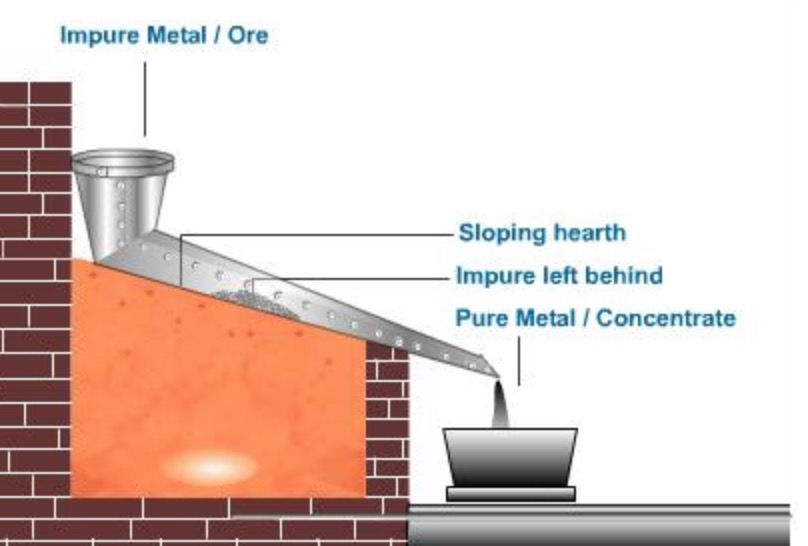
Liquation
- This technique is utilized for highly fusible metals, such as tin (with a melting point of 231.97°C) and lead (with a melting point of 327.5°C), among others.
- The process involves the placement of impure metals within a carefully inclined bed of a furnace, where they are subjected to controlled heating until the metal reaches its melting point. Upon undergoing the process of melting, the substance gracefully transitions into a liquid state, effortlessly cascading into a designated receptacle.
- In this elegant transformation, the non-fusible impurities, namely oxides, are left behind, unable to partake in the fluidity that characterizes the molten substance.
Zone Refining
- This technique is a specialized method employed for the purification of metals. This method entails the purification of metals to a significantly elevated level.
- A metal rod that is not pure is introduced into a vessel that is filled with an inert gas. Subsequently, an annular heating device is positioned around the upper section of the rod in order to apply heat to the metal of inferior quality.
- When the heater transitions to the subsequent zone, the pure metal undergoes a cooling process and undergoes crystallization.
- The impurities that undergo melting will be transported in conjunction with the motion of the heating element and subsequently transferred to the adjacent region. Subsequently, the aforementioned impurities are accumulated within the final zone, facilitating their subsequent separation.
Chromatography Method
- This approach relies on the utilization of chromatography. Chromatography primarily focuses on the various degrees of absorption by an absorbent material as well as the different rates of movement that components within a mixture exhibit.
- The chromatographic technique involves the placement of a metal sample containing impurities into a medium, which can be either liquid or gaseous. Subsequently, the medium is passed through an absorbent material.
- Various components will exhibit varying levels of absorption for the impure metal. Subsequently, the aforementioned components are extracted through the utilization of an appropriate solvent.
- Various chromatographic methods exist, including Column Chromatography, Thin layer chromatography, and Gas-Liquid Chromatography, among others.
References
- https://byjus.com/chemistry/refining/#:~:text=Refining%20is%20a%20method%20of,Distillation
- https://www.geeksforgeeks.org/what-is-refining-of-metals/
- https://biologyreader.com/refining-of-metal.html
- https://www.britannica.com/science/metallurgy/Refining
- https://collegedunia.com/exams/refining-of-metals-zone-refining-distillation-and-chromatographic-method-science-articleid-311
- https://www.embibe.com/exams/refining-or-purification-of-impure-metals/
