Raman spectroscopy is a type of vibrational spectroscopy that allows for the simple interpretation and very sensitive structural identification of trace amounts of substances based on their distinctive vibrational properties. Sir C.V Raman, an Indian physicist, demonstrated Raman spectroscopy, which is based on the inelastic scattering of monochromatic light with the sample. Because of the inelastic scattering, the resultant light will have a different frequency than the incident light.
This method is commonly used to investigate vibrational, rotational, and other low-frequency interactions in molecules. This is quite beneficial for identifying molecular structure, identifying functional groups, etc.
Because of its diagnostic potential, Raman spectroscopy has become a commonly utilized instrument in biomedical engineering and life science. It is used in medical research to examine the biochemical environment of single cells or to monitor cell reactions to medications, as well as in the pharmaceutical sector for process and quality control in drug manufacturing.
Interesting Science Videos
Raman effect
Raman spectroscopy is founded on the Raman effect, which was discovered in 1928 by Indian physicist Chandrasekhara Venkata Raman. The Raman effect is based on light scattering, which comprises elastic (Rayleigh) scattering at the same wavelength as the incident light as well as inelastic (Raman) scattering at various wavelengths caused by molecular vibrations. Rayleigh scattering is a million times more intense than Raman scattering. To acquire Raman spectra, Rayleigh scattering must be prevented from overwhelming the weaker Raman scattering.
Raman spectra are obtained by stimulating a sample with a high-intensity laser beam and passing the scattered light through a spectrometer. The Raman shift is the difference in energy between the incident and dispersed light. The vertical axis of the resulting spectrum represents the intensity of the scattered light, and the horizontal axis is the wavenumber of the Raman shift (cm-1).
Raman shift
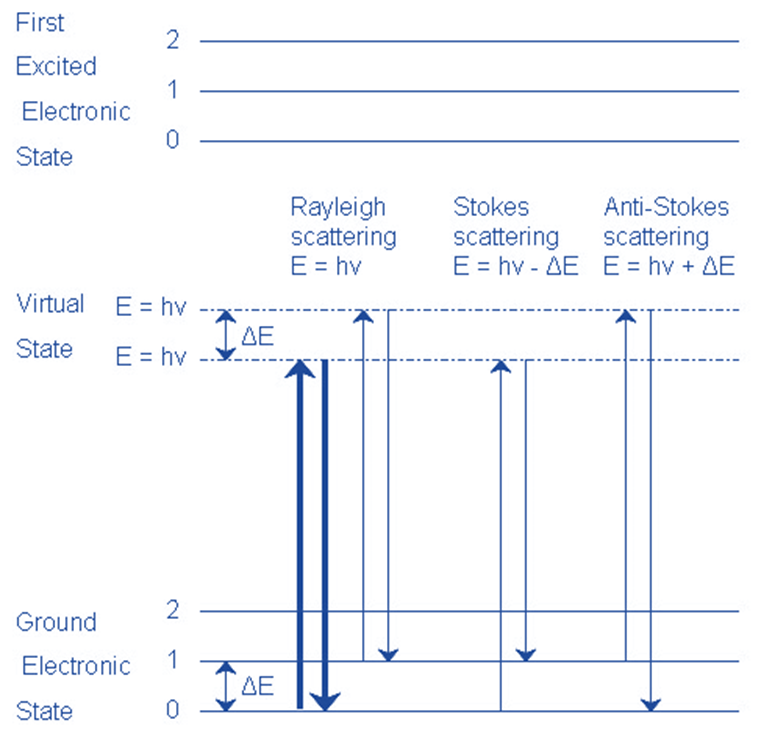
The Raman shift corresponds to two distinct energy bands. Stokes scattering refers to the shift at wavelengths greater than those of the incident light. Anti-Stokes scattering refers to the shift at wavelengths shorter than those of the incident light.
Principle of Raman spectroscopy
Raman spectroscopy is based on the inelastic scattering of electromagnetic light by a molecule. There are two forms of scattering: elastic and inelastic scattering.
Elastic scattering follows the Rayleigh law, which asserts that no energy or momentum is lost between the incident and dispersed radiation. As a result, this scattering is also referred to as Rayleigh scattering. However, there will be certain (1 in a million) cases when the energy of the dispersed radiation differs from the energy of the incidence radiation. This is known as inelastic scattering.
Raman spectroscopy uses inelastic scattered light to identify an analyte through molecular bond vibrations. A limited number of photons are dispersed when a sample is subjected to laser light. The majority of the scattering is elastically dispersed light with the same frequency as the incident light (i.e., Rayleigh scattering). Approximately one out of every 106-108 photons is inelastically scattered (i.e., Raman scattering), causing frequency differences (i.e., Raman shifts) between incident and scattered photons when energy is exchanged between photon and molecule. In this case, incident light photon energy is acquired by transferring energy from molecules to photons (anti-Stokes Raman scattering) or lost by transferring energy from photons to molecules (Stokes Raman scattering).
Raman spectra are generated using the measured Raman shifts. Each Raman peak in the spectrum corresponds to a single chemical bond, permitting molecular identification of an analyte by producing a unique vibrational fingerprint.
Rayleigh Scattering
- The molecule is stimulated to any virtual state.
- The molecule returns to its former state by relaxing.
- The photon is elastically scattered, retaining its initial energy.
Stokes Scattering
- The molecule is stimulated to any virtual state.
- The molecule returns to a higher vibrational state than it was before.
- The photon has been scattered inelastically with energy hv- ΔE.
Anti-Stokescattering
- In its first state, the molecule is vibrationally excited.
- The molecule is stimulated to any virtual state.
- The molecule returns to a lower vibrational state than it had previously.
- The photon escapes with energy hv+ ΔE and is superelastically scattered.
How does Raman Spectroscopy work?
Unlike FTIR spectroscopy, which examines variations in dipole moments, Raman examines changes in the polarizability of a chemical bond. Light interaction with a molecule can cause its electron cloud to distort. This deformation is known as polarizability change. Molecular bonds have unique energy transitions that cause polarizability to vary, resulting in Raman active modes. When photons interact with molecules that include bonds between homonuclear atoms, such as carbon-carbon, sulfur-sulfur, and nitrogen-nitrogen bonds, their polarizability changes. These are instances of bonds that give rise to Raman active spectral bands but that are either invisible or difficult to see in FTIR.
Because Raman is a weak effect by nature, the optical components of a Raman Spectrometer must be carefully matched and optimized. Furthermore, as organic molecules have a higher tendency to fluoresce when exposed to shorter wavelength radiation, longer wavelength monochromatic excitation sources, such as solid-state laser diodes emitting light at 785 nm, are commonly utilized.
Instrumentation of Raman spectroscopy

Fig: Instrumentation of Raman Spectroscopy
Image source: https://www.sciencedoze.com/2022/10/raman-spectroscopy-principle.html
- Source of excitation (laser)
A laser is used as a light source in Raman spectroscopy. The bandwidth of the utilized laser source determines the radiation’s spectrum. In general, a shorter wavelength produces more Raman scattering. Because Raman scattering intensity varies as the fourth power of frequency, argon, and krypton ion sources emitting in the blue and green regions of the spectrum have an advantage over other sources.
- Sample
Laser is irradiated on the sample present in the sample chamber. After interacting with the laser beam it gives elastic and inelastic scattering before passing through the filter.
Liquid Samples:
A significant advantage of Raman spectroscopy sample handling over infrared is that water is a poor Raman scatterer but a powerful absorber of
infrared radiation. As a result, aqueous solutions can be examined using Raman spectroscopy but not infrared.
This benefit is especially essential for biological and inorganic systems, as well as studies dealing with water pollution issues.
Solid samples
Solid-state Raman spectra are frequently obtained by filling a tiny cavity with the sample after it has been ground to a fine powder. Polymers are typically evaluated immediately without sample preprocessing.
Gas samples
Gas is often contained in glass tubes that are 1-2 cm in diameter and around Imm thick. Small capillary tubes can also be used to seal gases.
- Filter
In Raman spectroscopy, a filter is employed to separate Raman scattered light from rayleigh scattered light. This is done in order to obtain high-quality Raman spectra. Notch, long pass, and volume halogen filters are examples of filters.
- The detector
The detector aids in the detection of the scattered light signal. LCD array detectors are commonly employed in current Raman spectrometers. They are designed to detect signals of various wavelengths, as well as very weak signals.
- Computer
A computer with relevant software aids in the creation of a final Raman spectroscopy graph/ spectrum.
Types of Raman spectroscopy
Surface-enhanced Raman spectroscopy (SERS)
Surface-enhanced Raman spectroscopy (SERS) is one of the most sensitive devices, allowing for highly sensitive structural detection of low-concentration analytes via the amplification of electromagnetic fields generated by the excitation of adsorbate molecules’ localized surface plasmons (LSP) on a roughened metal surface.
Surface-enhanced hyper Raman Scattering (HR) scattering
Surface-enhanced hyper Raman scattering is an inelastic sum of frequency scatted from two photons, whereas normal Raman scattering results from a single photon. SERS’s total surface enhancement factors are expected to be in the order of 1014. The amplification could be due to molecular electronic resonance, in which the molecular transition corresponds with the sum frequency of the input photons.
Tip enhanced Raman spectroscopy (TRES)
Tip enhanced Raman spectroscopy (TERS) is a strong technology that combines two techniques: scanning probe microscopy and Raman spectroscopy. TERS can give topographical and spectral/chemical information at the same time by utilizing SPM and Raman.
TERS uses a metal tip or metal nanoparticle instead of a metal film and has identical instruments, material requirements, and enhancement principles as SERS.
Coherent anti-Stokes Raman Spectroscopy (CARS)
Coherent anti-stoke Raman scattering spectroscopy is a nonlinear Raman technique used to amplify the Raman signal. It employs coherent laser beams to generate a signal with a frequency greater than the excitation frequency and is thus classified as an anti-stoke frequency approach.
Resonance Roman spectroscopy (RRS)
Resonance Raman spectroscopy is a technique that measures the shift in photon frequency when the energy of photons from incident light is approximately similar to the energy required for electronic transition. Resonance excitation can increase electron oscillation charge displacement. As a result, it will raise the induced dipole moment, which instantly enhances Raman scattering efficiency.
Confocal Raman microscopy
Marvin Minsky invented the first confocal Raman microscopy in 1955. In Confocal Raman Microscopy, the probe head focuses laser light on the sample via the microscope objective. And the pinhole refocuses the backscattered Raman signal, causing it to act as a spatial filter. The signal will then be gathered by a charge-coupled device camera (detector) to generate a spectrum.
Applications of Raman spectroscopy
- Raman spectra are similar to infrared spectra in that they consist of functional group detection zones and fingerprint sections that allow specific compound identification. Raman spectra provide more information about certain types of organic molecules than infrared spectra.
- Raman spectra are less populated with peaks than infrared spectra. As a result, peak overlap in mixtures is less frequent, and quantitative measurements are more straightforward. Furthermore, Raman sampling equipment is not affected by moisture, and little amounts of water in a sample have no effect.
- Since aqueous solutions can be used, the Raman technique is frequently superior to infrared for the spectroscopy of inorganic systems.
- It is used to investigate the structure of CO2, N2O, mercurous salts, mercury chloro complexes, and the nature of bonding.
- It is useful for studying electrolytic dissociation, hydrolysis, and the transition from crystalline to amorphous states in physical chemistry.
- It is also used to determine the presence or absence of certain links in a molecule, as well as the structure of simple compounds and the study of isomers.
- Raman amplification is based on Raman scattering, in which lower-frequency photons are pumped to a high-frequency zone with an excess of energy. This method can be used in telecommunications.
- Raman spectroscopy is based on the Raman effect and has applications in many fields, including nanotechnology to study the structure of nanowires, biology, and medicine to study low-frequency DNAs and proteins, and chemistry to study the structure of molecules and their bonds.
- Remote sensing and planetary exploration make use of Raman scattering.
- When combined with optical fibers, Raman spectroscopy provides the added benefit of remote sensing. The optical fibers are responsible for transporting Raman signals by collecting scattered photons.
- Raman spectroscopy has been employed in real-time monitoring systems to detect illegal substances, harmful environmental materials, and chemical and biological warfare weapons.
Advantages of Raman Spectroscopy
- Because of its better spectral resolution and narrower bandwidths, Raman spectroscopy enables multiplex detection of various analytes.
- Raman works with any sample composition. Analyte concentrations in solvents, tissue, and solids (for example, drug composition) can all be measured.
- There is no requirement for sample preparation or particular markers (but they can be used in conjunction with Raman measurements).
- Raman can sense individual analytes on a subcellular level and determine their distribution in a cell when paired with microscopy.
- Raman can be easily combined with microfluidic devices to develop more complicated and automated analytical measurement setups.
- It is a non-invasive technology for subsurface sensing in tissue and measurements in vivo.
Comparison between Raman and IR spectroscopy
Although Raman and infrared spectroscopy are both based on molecular vibrations, as shown below.
IR spectroscopy
- The absorption of light energy corresponding to the vibrational energy of molecules is the basis for infrared spectroscopy.
- A vibration is Raman active if it induces a change in polarisability.
- The molecule does not require to have a constant dipole moment.
- Water can be used as a solvent.
- Sample preparation is simple; it can be in any state.
- It Indicates the presence of covalent bonds in the molecule.
- The cost of instrumentation is high.
Raman spectroscopy
- Raman spectroscopy is based on the scattering of incident light at an energy altered by the molecule’s vibrational energy (hv).
- Vibration is IR active if the dipole moment changes.
- The vibration in question should cause a change in the dipole moment.
- Water cannot be utilized due to its high IR absorption.
- The sample preparation is rigorous.
- Gaseous samples are rarely useful.
- Indicates the ionic nature of the molecule.
- Relatively affordable
Comparison between Raman spectroscopy and FTIR spectroscopy
Even though FTIR and Raman Spectroscopy provide complementary information and are frequently interchangeable, several practical considerations affect which one is the best choice for a particular experiment.
Raman spectroscopy is used
- When the primary focus of research is on carbon bonding in aliphatic and aromatic rings.
- For the bonds that are difficult to see in FTIR (for example, O-O, S-H, C=S, N=N, C=C, and so on)
- Polymorphism, for example, requires the examination of particles in solution.
- For lower frequency modes (for example, inorganic oxides) are relevant.
- To the study of reactions in aqueous media
- To investigate lower-frequency lattice modes.
- For investigation of biphasic and colloidal reaction initiation, endpoint, and product stability
FTIR is used
- For investigating liquid-phase reactions
- For Fluorescent reactions in which reactants, reagents, solvents, and reaction species
- Bonds having large dipole changes (e.g., C=O, O-H, N=O) are important.
- For reactions involving low concentrations of reagents and reactants
- For reactions with strong Raman solvent bands that can obscure crucial species signals
- For reactions in which the intermediates produced are IR active
References
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_%28Barron%29/04%3A_Chemical_Speciation/4.03%3A_Raman_Spectroscopy.
- https://www.slideshare.net/BhaumikBavishi/raman-spectroscopy-54641528.
- https://www.jasco-global.com/principle/1-what-is-raman-spectroscopy/.
- https://www.researchgate.net/publication/309179824_Raman_Spectroscopy_a_review.
- https://www.sciencedoze.com/2022/10/raman-spectroscopy-principle.htm
- https://byjus.com/physics/raman-scattering/.
- https://www.mdpi.com/2079-6374/11/6/187
