Radon is the last member of the noble gas family. The noble gases are the elements that make up Group 18 (VIIIA) of the periodic table. Radon has the atomic number 86. It is found in Group 18 and Period 6 of the periodic table of elements. It is represented by the symbol ‘Rn’. Radon is produced when heavier radioactive materials, such as uranium and thorium, decay. Radon, in turn, degrades to generate lighter elements like lead and bismuth.
Interesting Science Videos
History of Radon
- Ernest Rutherford detected a gas originating from a sample of thorium ore in 1899, while Pierre and Marie Curie identified a radioactive gas originating from radium in the same year.
- Fredrich E. Dorn discovered radon gas at Halle, Germany, in 1900. He dubbed it radium emanation because it originated from the element radium, which he was working with.
- William Ramsay and Robert Gray separated the gas and termed it niton in 1908.
- Since 1923, it has been known as radon (after radium, one of its origins).
- Radon was one of the first radioactive elements to be found, following uranium, thorium, polonium, and radium.
Occurrence of Radon
- Radon occurs naturally as a byproduct of the disintegration of uranium, radium, thorium, and other radioactive elements.
- The amount of radon in the atmosphere is too tiny to quantify. Radon is constantly present since it is generated during the breakdown of uranium and radium. It is constantly present outside and in natural-source drinking water, but at a low level in open places. It is more prevalent in enclosed settings, such as homes or mines.
- Radon is produced directly by the alpha-ray decay of an aqueous solution of radium chloride.
- Water, soil, and groundwater are all sources of radon. Concrete, granite worktops, and wallboards are other construction elements that also produce radon.
- Radon has 33 isotopes with confirmed half-lives, with mass numbers ranging from 196Rn to 228Rn. None of them are stable. With a half-life of 3.8 days, 222Rn is the most steady isotope.
Elemental Properties of Radon
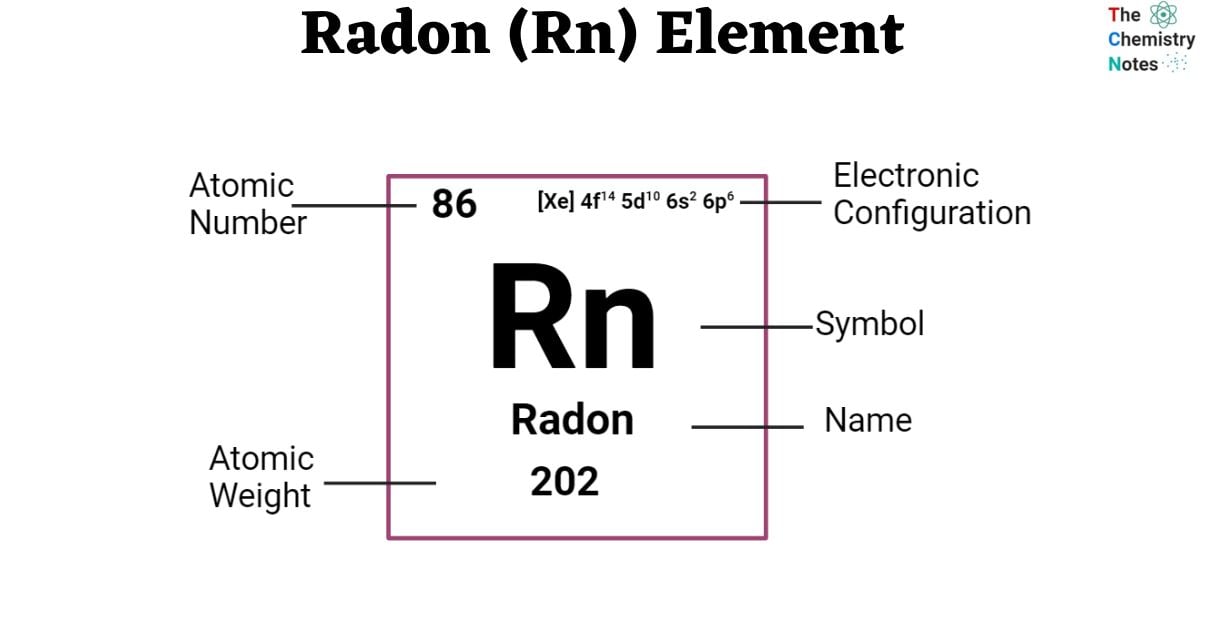
| Electronic Configuration | [Xe] 4f14 5d10 6s2 6p6 |
| Atomic Number | 86 |
| Atomic Weight | (222) g.mol -1 |
| State at 20°C | Gas |
| Group, Period, and Block | 18, 6, p-block |
| Density | 0.00973 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 32, 18, 8 |
| Electrons | 86 |
| Protons | 86 |
| Neutrons | 136 |
Physical Properties of Radon
- Radon has an atomic number of 86 and is a noble gas. It has a melting point of (−71 °C, −96 °F) and a boiling point of (−61.7 °C, −79.1 °F).
- Radon has a density of 9.72 grams per liter, which is approximately seven times that of air. It is the densest known gas.
- Compared to other noble gases, radon is more soluble in water. Additionally, compared to water, it is more soluble in organic liquids.
- At normal pressure and temperature, radon is colorless, odorless, and tasteless.
- Radon dissolves in water and creates a clear, colorless liquid under its boiling point. Liquid radon freezes at much colder temperatures. As a solid, its hue undergoes a shift from yellow to orangish-red as the temperature descends even more.
- Due of the high radiation produced, radon liquid glows.
| Color/physical appearance | Colorless, Odorless, Tasteless |
| Melting point/freezing point | 202 K (−71 °C, −96 °F) |
| Boiling point | 211.5 K (−61.7 °C, −79.1 °F) |
| Density | 0.009074 g/cm3 at 20° |
| Flammability | Not Flammable |
| State of matter at room temperature | Gas |
Chemical Properties of Radon
- Radon is not a particularly reactive gas.
- Strong oxidizing substances are capable of oxidizing radon.
- Because of the exorbitant expense of research and the radioactivity of radon, its chemical composition has received little attention.
Chemical Reaction of Radon
- The Reaction of Radon with Water
Radon gas does not react with water.
- The Reaction of Radon with Air
Radon gas does not react with air.
- The Reaction of Radon with Halogens
Other than fluorine(F), radon gas does not react with other halogens. Radon gas appears to react with fluorine to form the difluoride radon(II) fluoride, RnF2, however the chemistry has not been well investigated.
- The Reaction of Radon with Acids
Radon gas does not react with acids.
Uses of Radon
Radon has no large-scale industrial uses, however, it does have the following:
- Radon released by a radium source can be utilized to treat cancer. It was extensively employed in hospitals to treat malignancies by implanting minute tubes into which the gas was trapped. It has now been substituted by safer therapy methods.
- Researchers utilize radon soil concentrations to identify underlying geological faults because concentrations typically rise above the faults. Groundwater radon concentrations are often monitored in an attempt to anticipate earthquakes.
- Radon testing kits are used to assess indoor radon levels in areas where high amounts of radon gas are likely to accumulate. If the test result indicates a level of 4 pCi/L or more, mitigation techniques are utilized to minimize radon concentrations.
- The radioactive breakdown of radon can be used to create polonium.
Health Hazards of Radon
- Exposure to high quantities of radon by inhaling air is known to induce lung illnesses. Long-term exposure to radon raises the risk of getting lung cancer. Radon may only cause cancer after years of exposure.
- Radon is radioactive, yet it emits minimal gamma radiation. As a result, negative effects from radon radiation without direct interaction with radon compounds are unlikely.
- It is unknown if radon may harm organs other than the lungs. The consequences of radon, which can be present in food or drinking water, remain unclear.
Environmental Hazards of Radon
- Radon is a radioactive substance that occurs infrequently in nature. The majority of radon chemicals detected in the environment are the result of human activity.
- Radon enters the environment via the soil, uranium and phosphate mines, and coal combustion.
- When released by renewable energy sources such as geothermal power plants, radon is a known contaminant, yet it has little influence on our planet’s ecosystem or human health.
Video Reference
References
- https://www.chemistrylearner.com/radon.html
- https://www.rsc.org/periodic-table/element/86/radon
- https://byjus.com/chemistry/radon/
- https://www.chemicool.com/elements/radon.html
- https://pandakajal42.medium.com/radon-element-properties-effects-facts-6a21806f2f4e
- https://www.lenntech.com/periodic/elements/rn.htm
- https://www.webelements.com/radon/chemistry.html
- https://chemicalengineeringworld.com/radon-element-properties-and-information/

