
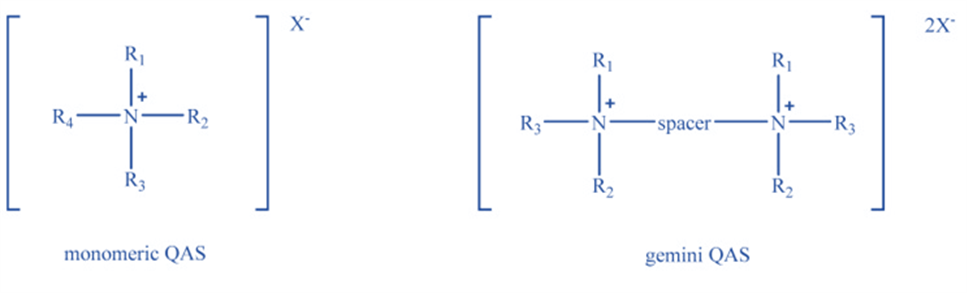
Quaternary ammonium salts are chemical compounds with the formula NR+4X–, where R is an alkyl or aryl group and X is an anion (typically a halide ion). The quaternary ammonium cations are always positively charged. In aliphatic salt, the central nitrogen atom is substituted with alkyl or alkyl aryl groups. Depending on the kind of substituent, the salts can be classified as alkylammonium, alkylmethylammonium, alkyl dimethylammonium, alkyltrimethylammonium, alkylbenzyldimethylammonium, or dialkyldimethylammonium.
In addition to aliphatic salts, the category of chemicals classed as quaternary ammonium salts includes a vast number of salts that are heterocyclic derivatives, such as pyridinium, imidazolinium, imidazolium, benzimidazolium, quinoline, isoquinoline, piperidinium, morpholinium, and benzamidinium.
Because quaternary ammonium halides lack an unshared electron pair on the nitrogen atom, they cannot operate as bases. However, quaternary ammonium hydroxides are strong bases.
They are made up entirely of hydroxide ions (OH-) and quaternary ammonium cations (R4N+), whether in solution or as solids. So, quaternary ammonium hydroxide has the same potency as sodium or potassium hydroxide.
What are Quaternary ammonium salts?
Quaternary ammonium salts (QAS) are ionic compounds composed of quaternary ammonium nitrogen, four alkyl or aryl groups linked to this nitrogen, and an anionic ion such as chloride or bromide. One of the four alkyl groups is a long alkyl chain group with more than eight hydrocarbons that also functions as a hydrophobic group.
Quaternary ammonium (QA) salts are highly stable chemicals with phase transfer catalytic and germicidal properties. Hydrophobic groups on Quaternary ammonium salts have been shown to have an impact on their antibacterial activities. All of these chemicals are membrane-active and can destroy gram-positive bacteria cell walls. When employed in aqueous solutions and as liquid disinfectants, Quaternary ammonium salts are effective biocides.
When Quaternary ammonium salts are chemically attached to fiber surfaces, their functions can be hampered depending on how they are coupled and the final frameworks of QAS on surfaces. Physically embedded QAS in fibers can give antimicrobial capabilities by gradually releasing them from the fiber’s surfaces throughout use, which could meet the materials’ intended functions but may pose a durability risk.
Quaternary ammonium compounds are a type of chemical that is used to kill bacteria, viruses, and mold. QACs come in a variety of shapes and sizes. They are commonly found in disinfectants and cleaning solutions used in hospitals, daycare centers, restaurants, and households.
Preparation of quaternary ammonium salt
A tertiary amine was combined with four commercially available alkyl halides bearing carboxylic or organosilane functional groups to produce quaternary ammonium ions. A Menschutkin reaction occurs when a neutral amine reacts with an alkyl halide to produce two ions of opposing sign. It’s an SN2 (bimolecular nucleophilic substitution) reaction. This reaction takes place on an aliphatic carbon that has a stable, electronegative leaving group (halide). During the transition state, the carbon-halide link is broken and a new nitrogen-carbon bond is formed at the same time. Final products in solutions might exist as single ions, ion pairs, or solid salts.
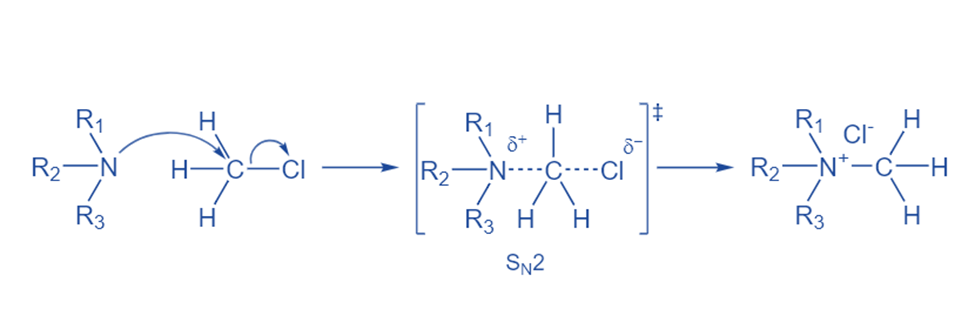
So, the Menshutkin Reaction involves the reaction of a tertiary amine with an alkyl halide to produce a quaternary ammonium salt.
Applications of quaternary ammonium salt
- Quaternary ammonium salts are ubiquitous ingredients in cleaning goods, although there is growing worry due to increased antibiotic resistance.
- Quaternary ammonium salt monomers with antimicrobial properties can limit bacterial activity in dental resins (bio-active function). They had antibacterial activity before polymerization and did not release any antibacterial components while keeping their contact-inhibitory properties.
Olefin Synthesis
When quaternary ammonium hydroxide is heated, a 13-elimination reaction occurs, yielding an alkene that distills from the reaction mixtures. Hofinann elimination is the name given to this sort of elimination reaction.
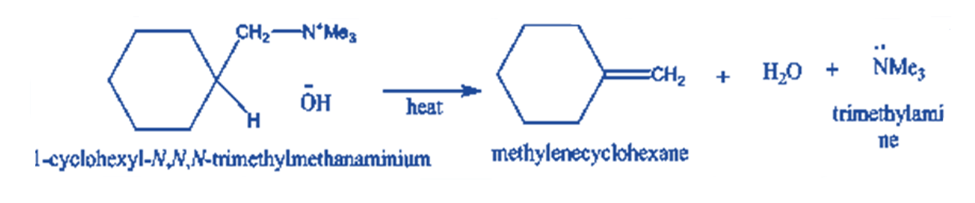
Because quaternary ammonium halides do not undergo Hofmann elimination, they are transformed into quaternary ammonium hydroxides, which are then Hofmann eliminated to generate alkenes.
Displacement reaction
Bulky quaternary salts, such as tetrabutly- or higher tetraalkylammonium salts, perform exceptionally well in displacement processes. The capacity to activate anions is due to their increased solubility in the organic phase.
The commercial use of cyanide displacement is the conversion of 1,6-dichlorohexane to suberonitrile, which is used as an intermediary in the synthesis of 1,8-diaminooctane and suberic acid, which is used for making Nylon-8.

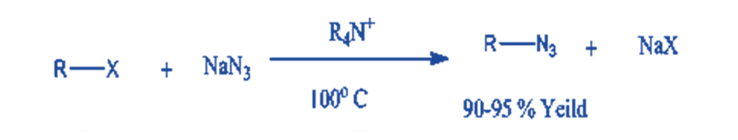
Azide displacement reactions with n-octyl chloride and bromide utilizing tetrabutylammonium halides result in high yields of alkyl azides.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6515548/#:~:text=Quaternary%20ammonium%20salts%20were%20synthesized,(AMsil%20series)%20functional%20groups.
- https://link.springer.com/chapter/10.1007/978-94-009-0023-3_3.
- https://www.sciencedirect.com/topics/chemistry/quaternary-ammonium-salt.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7350379/
