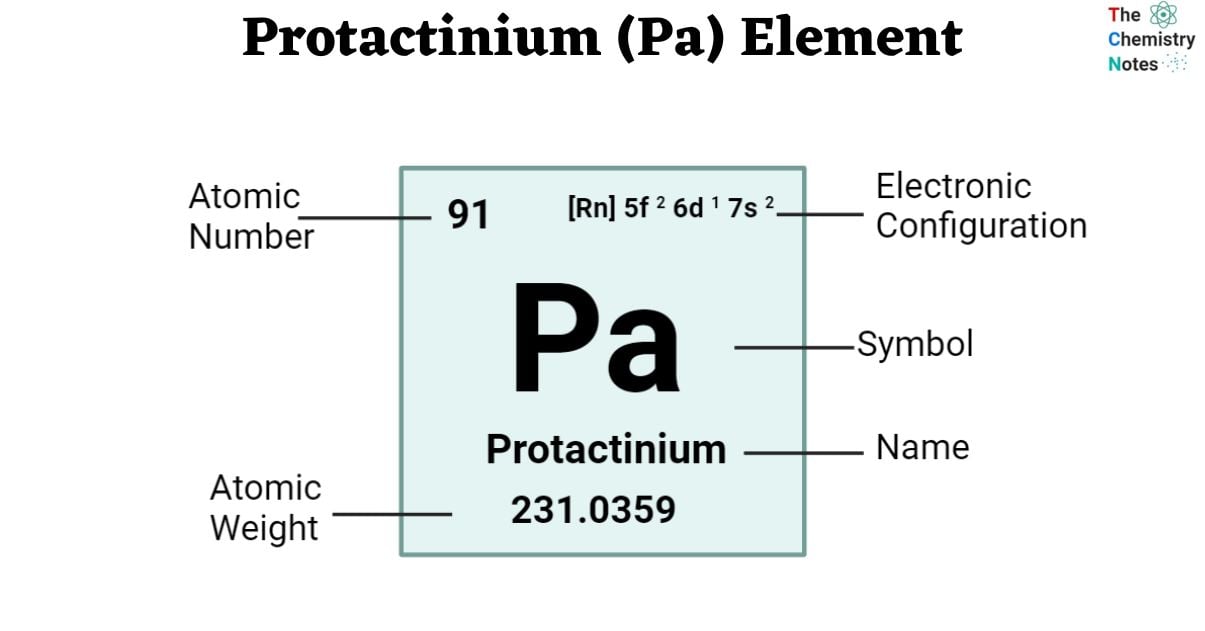
The atomic number of protactinium is 91. It is found in f-block and Period 7 of the periodic chart. The symbol ‘Pa’ represents it. The name derives from word “Protos,” which translates to “first,” is combined with the element “Actinium,” a byproduct of protactinium’s disintegration. It is soft, silvery-white in appearance. Protactinium normally exists in the oxidation state +5, while it may also take on +4, +3, or +2 states, as it forms a variety of chemical combinations.
The naturally occurring element protactinium is very costly and scarce. This metal and its derivatives are poisonous and radioactive, making them hazardous to handle.
Interesting Science Videos
History of Protactinium
- Dmitri Mendeleev predicted the existence of an element between thorium and uranium on the periodic table. However, the actinide group was unknown at the time.
- William Crookes extracted protactinium from uranium in 1900, but he was unable to describe it, therefore he is not credited with its discovery.
- In 1913, Polish chemist Kasimir Fajans and German chemist Oswald Göhring discovered protactinium in Karlsruhe, Germany. They named the element ‘brevium’ because the isotope they discovered (protactinium-234) had a relatively short half-life (1.17 minutes).
- John Cranston and Frederic Soddy, two scientists from the University of Glasgow in Scotland, discovered protactinium on their own in 1917.
- Aristid von Grosse discovered protactinium oxide (Pa2O5) in 1927. In 1934, he separated the element from the oxide by converting it to iodide (PaI5) and then decomposing it in an intense vacuum employing a hot filament.
Occurrence of Protactinium
- Protactinium is a rare naturally occurring element. It is also the most costly ingredient of all. It can be discovered in most natural materials, where it is distributed in unextractable levels.
- It occurs naturally in extremely small amounts (on the order of 1 ppt or 0.1 picocuries (pCi)/g) in soil, rock, surface water, groundwater, plants, and animals.
- It is found in trace concentrations (up to 3 parts per million) in uranium ores. It can also be produced as a byproduct of uranium processing.
- Uraninite contains ‘Pa’ at concentrations of 0.3 to 3 parts 231Pa ppm. Some ores from the Democratic Republic of the Congo contain a composition of about 3 ppm, compared to the average of about 0.3 ppm.
Isotopes of Protactinium
- Protactinium has 27 isotopes having known half-lives and mass numbers ranging from 212Pa to 238Pa. There are no stable isotopes of protactinium.
- The isotopes with longest half-lives are 231Pa, which has a half-life of 32,5280 years, 233Pa, which has a half-life of 26.967 days, and 230Pa, which has a half-life of 17.4 days.
- The first isotope found was 234Pa, often known as UX2.
- 234Pa is a short-lived member of the U-238 decay series, which occurs naturally.
- Hahn and Meitner detected the longer-lived isotope, 231Pa, in 1918.
Elemental Properties of Protactinium
| Electronic Configuration | [Rn] 5f 2 6d 1 7s 2 |
| Atomic Number | 91 |
| Atomic Weight | 231.0359 g.mol -1 , no stable isotope |
| State at 20°C | Solid |
| Group, Period, and Block | 7, f-block |
| Density | 15.4 g/cm3 at 20 °C |
| Ionic radius | unknown |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 32, 20, 9, 2 |
| Electrons | 91 |
| Protons | 91 |
| Neutrons in most abundant isotope | 140 |
Physical Properties of Protactinium

[Image source: wikipedia]
- ‘Pa’ is a rare and costly naturally occurring element.
- Protactinium is a radioactive element of the actinide family. It has a silver-grey color and is thick.
- Pa has an atomic number of 91. It has a melting point of 1568 °C (2854 °F) and a boiling point of 4027 °C (7280 °F).
- Protactinium has qualities that are midway between thorium and uranium.
- Below the temperature of 1.4K (-271.75 °C), the element is superconductive.
- Protactinium is an alpha emitter (5.0 MeV) and a radioactive danger that must be handled carefully.
- ‘Pa’ is a paramagnetic element.
- ‘Pa’ crystallizes in a body-centered tetragonal form.
- At ambient temperature, protactinium crystallizes in the body-centered tetragonal structure, which may be seen of as a deformed body-centered cubic lattice; this structure does not alter during compression up to 53 GPa.
| Color/physical appearance | Lustrous, silvery-grey |
| Melting point/freezing point | 1841 K (1568 °C, 2854 °F) |
| Boiling point | 4300 K (4027 °C, 7280 °F) |
| Density | 15.37 g/cm3 at 20° |
| Malleability | Yes |
| Ductility | N/A |
| Electronegativity | 1.5 (Pauling Scale) |
Chemical Properties of Protactinium
- Protactinium compounds have oxidation states of +4 and +5. This is true for both solutions and solids.
- In solution, the +5 oxidation state quickly reacts with hydroxide ions to generate (radioactive) hydroxy-oxide solids that adhere to the container’s surface.
- Some solid-phase protactinium compounds exhibit oxidation states of +3 and +2.
- Oxides, hydrides, halides, and organometallic compounds have been found to be formed from protactinium.
- When exposed to oxygen, it rapidly combines to create a white coating of protactinium oxide.
- Protactinium combines with the halogens (fluorine, chlorine, bromine, and iodine) and with hydrogen to produce compounds. However, these chemicals have not been thoroughly investigated.
Uses of Protactinium
Protactinium and its derivatives have no practical applications in industry. It is now only used in fundamental scientific study because to its dearth, high radioactivity, and toxicity. Some of its uses and potentials are mentioned here:
- It might be used to model the mineral production and glacier melting processes.
- It also can be used in order to ensure that sediments may be radiometrically dated for up to 175000 years, modern mass spectrometers can detect the ratio of 231Pa to thorium-230.
Toxicity of Protactinium
- Protactinium is typically only harmful to humans if it is ingested; nevertheless, protactinium-231’s gamma rays and a handful of actinium-227’s short-lived decay products provide a modest external danger.
- Protactinium-containing food, drink, and inhaled protactinium-contaminated dust are the primary exposure routes.
- Unless there is a nearby source of contaminated airborne dust, ingestion is usually the cause of concern.
- Ionizing radiation from protactinium that has been accumulated in the skeleton, liver, and kidneys is the main health issue since it can cause cancer.
- The health concerns connected with protactinium-234m are the same as those relating to uranium-238. Because protactinium-234m decays by producing an energetic beta particle, precautions against this radiation are required while working with uranium, such as wearing strong rubber gloves to protect the hands and forearms.
Environmental Hazards of Protactinium
- Protactinium adheres to soil extremely firmly, and the concentration associated with sandy soil particles is roughly 550 times larger than in interstitial water concentration ratios for loam and clay soils, which are substantially higher at 2,000 and above.
Video Reference
References
- https://www.rsc.org/periodic-table/element/91/protactinium
- https://collegedunia.com/exams/protactinium-properties-effects-and-solved-examples-articleid-5070
- https://www.chemicool.com/elements/protactinium.html
- https://www.lenntech.com/periodic/elements/pa.htm
- https://www.americanelements.com/pa.html
- https://www.thoughtco.com/pa-element-or-protactinium-facts-606582
- https://chemicalengineeringworld.com/protactinium-element-properties-and-information/
- https://www.chemistrylearner.com/protactinium.html

