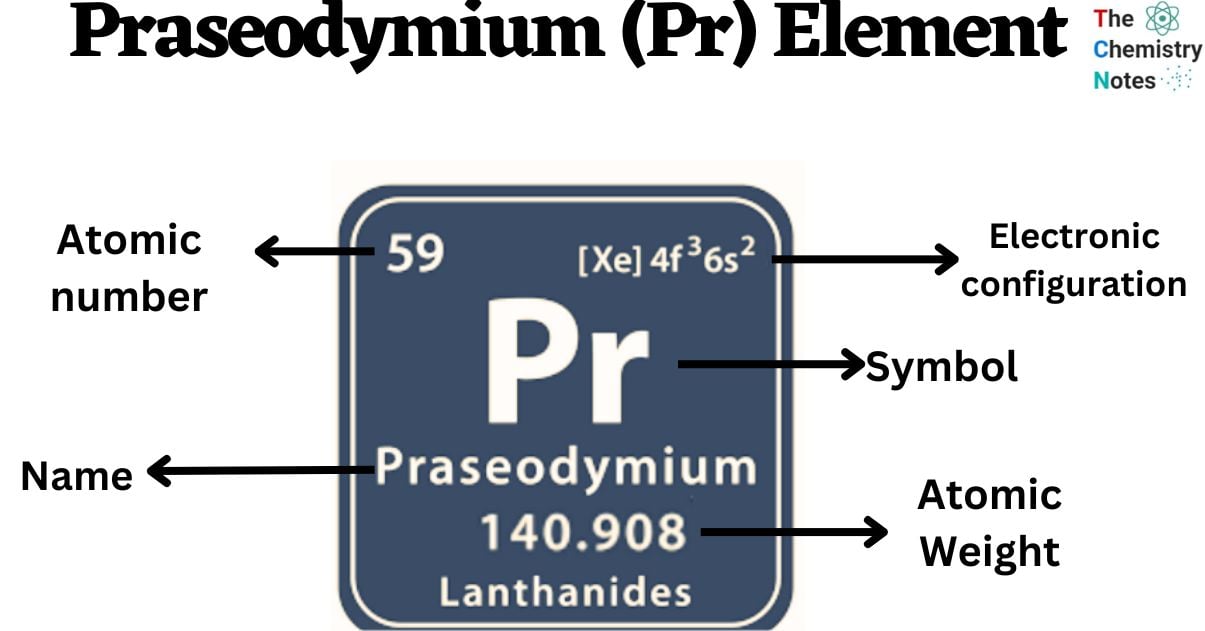
Praseodymium is a chemical element with the atomic number 59 and is represented by the symbol ‘Pr’ in the periodic table. It is soft and silvery in appearance and classified as rare earth metal and belongs to the f-block of the lanthanide group of the periodic table. Similar to other rare-earth elements, praseodymium exhibits a strong tendency to adopt the +3 oxidation state. This particular state remains stable in aqueous solutions, while the +4 state of oxidation is also observed in certain solid compounds. Interestingly, praseodymium stands out among the lanthanides as it is capable of achieving the +5 oxidation state under matrix-isolation conditions.
Praseodymium can be found in the Earth’s crust with a typical concentration of 8 ppm (parts per million). Pr is not rare, as it is found in a variety of minerals, although the minerals belonging to the monazite and bastnäsite groups are considered the most significant sources in commercial applications.
Interesting Science Videos
History of Praseodymium
- Didymium, which Carl Mosander wrongly identified as a new element in 1841, was the source of this discovery.
- In the year 1879, the French chemist Lecoq de Boisbaudran successfully identified and isolated the element samarium from the compound known as ‘didymium’.
- Following the discovery of samarium, it was observed that the absorption spectrum of ‘didymium’ exhibited variations depending on the specific mineral from which it was derived.
- In the year 1885, Carl Welsbach, the individual credited with the discovery of ‘didymium’ fourteen years prior, came upon the realization that it was, in fact, a composite of two distinct and novel elements. The elements were given the names praseodymium and neodymium.
- As a nod to its green salts and its close relationship to neodymium, the element was given the Greek name “prasios didymos,” which translates to “green twin.”
Occurrence of Praseodymium
- Praseodymium is commonly present in the Earth’s crust at an average concentration of 8 parts per million (ppm).
- Praseodymium is not naturally occurring in its elemental form but rather exists within various minerals, primarily monazite, and bastnaesite.
- Commercial recovery of the substance is achieved through the utilization of ion exchange techniques and counter-current liquid-liquid extraction processes, which are employed on monazite sand and bastnaesite.
- Praseodymium metal can be obtained through the reduction of its anhydrous chloride compound.
- In total, there are 32 known isotopic forms of praseodymium, ranging in mass numbers from 121 to 154, and their respective half-lives have been determined. Praseodymium in its natural state is composed solely of the stable isotope 141Pr.
Isotopes of Praseodymium
Praseodymium only consists of one naturally occurring stable isotope: 141Pr.
| Isotopes | Natural abundance (atom %) |
|---|---|
| 141Pr | 100 |
Elemental Properties of Praseodymium
| Electronic Configuration | [Xe] 4f3 6s2 |
| Atomic Number | 59 |
| Atomic Weight | 140.9077 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 6, f-block |
| Density | 6.770 g.cm -3 at 20 °C |
| Ionic radius | 0.101 nm (+3) |
| Van der Waals radius | unknown |
| Electron shells | 2, 8, 18, 21, 8, 2 |
| Electrons | 59 |
| Protons | 59 |
| Neutrons in most abundant isotope | 82 |
Physical Properties of Praseodymium

- Praseodymium has an atomic number of 59 and is a silvery-white rare earth metal. It has a melting point of 935 °C (1715 °F) and a boiling point of 3130 °C (5666 °F).
- Pr has a solid phase density of 6.770 g/cm3 and a liquid or molten phase density of 6.50 g/cm3.
- It is malleable which means it can be easily beaten into thin sheets without any cleavage.
- It is ductile which means it is possible to draw thin wires from it without breaking.
- Praseodymium, like other early trivalent lanthanides, exhibits a double hexagonal close-packed crystal structure at room temperature.
- At approximately 560 °C, a transition occurs wherein the structure changes to a face-centered cubic arrangement, followed by the appearance of a body-centered cubic structure shortly before reaching the melting point of 935 °C.
| Color/physical appearance | metallic, silvery-white |
| Melting point/freezing point | 1208 K (935 °C, 1715 °F) |
| Boiling point | 3403 K (3130 °C, 5666 °F) |
| Density | 6.770 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 1.13 (Pauling Scale) |
Chemical properties of Praseodymium
- Praseodymium, upon exposure to air, undergoes a gradual process of tarnishing by which layers of spalling oxide are formed, reminiscent of the phenomenon observed in the rusting of iron. When praseodymium is exposed to air for extended durations, it is susceptible to complete corrosion.
- The element praseodymium exhibits a propensity to combust at relatively low temperatures of approximately 150°C, resulting in the formation of praseodymium(III, IV) oxide. It is worth noting that this compound is classified as non-stoichiometric due to its unique composition.
- Many different phases of praseodymium oxides occur as disordered non-stoichiometric phases when heated to 1000 degrees Celsius.
- The element praseodymium exhibits a chemical reaction when it comes into contact with water, resulting in the formation of praseodymium hydroxide alongside the liberation of hydrogen gas.
- Praseodymium predominantly manifests as a trivalent ion, denoted as Pr3+, within its various chemical compounds. The majority of its salts exhibit a delicate pale green hue.
- The element praseodymium exhibits a remarkable propensity to engage in chemical reactions with various halogens, resulting in the formation of compounds known as trihalides.
Chemical Reaction of Praseodymium
- The Reaction of Praseodymium With Air
Under ordinary circumstances, praseodymium exhibits a gradual reaction with oxygen, specifically O2, resulting in the formation of a tarnished surface. Praseodymium, when subjected to combustion, undergoes oxidation and transforms into a combination of praseodymium oxides, characterized by the approximate chemical formula Pr6O11.
12 Pr (s) + 11 O2 (g) [when heated] Δ → 2Pr6O11 (s)
- The Reaction of Praseodymium With Water
Praseodymium engages in a gradual reaction with cold water, while its reactivity is significantly enhanced when exposed to warm water. This reaction yields praseodymium hydroxide, denoted as Pr(OH)3, along with the liberation of hydrogen gas (H2).
2 Pr (s) + 6 H2O (g) → 2 Pr(OH)3 (aq) + 3 H2 (g)
- The Reaction of Praseodymium With Halogens
The element praseodymium exhibits a remarkable propensity to engage in chemical reactions with various halogens, resulting in the formation of praseodymium (III) halides.
The reaction between praseodymium metal and fluorine gas (F2) results in the formation of praseodymium (III) fluoride, denoted as PrF3.
2 Pr (s) + 3 F2 (g) → 2 PrF3 (s) [green]
The reaction between praseodymium metal and bromine (Br2) yields praseodymium (III) bromide (PrBr3).
2 Pr (s) + 3 Cl2 (g) → 2 PrCl3 (s) [green]
Praseodymium metal undergoes a reaction with chlorine gas (Cl2), resulting in the formation of praseodymium (III) chloride (PrCl3).
2 Pr (s) + 3 Br2 (g) → 2 PrBr3 (s) [green]
The reaction between praseodymium metal and iodine (I2) results in the formation of praseodymium (III) iodide, denoted as PrI3.
2 Pr (s) + 3 I2 (g) → 2 PrI3 (s)
- The Reaction of Praseodymium With Acids
Praseodymium metal is easily dissolved in dilute sulphuric acid, resulting in the formation of solutions including an aquated Pr(III) ion, which appears green, along with the liberation of hydrogen gas, H2. Pr3+(aq) is predominantly present in the form of the complex ion [Pr(OH2)9]3+.
2 Pr (s) + 3 H2SO4 (aq) → 2Pr3+ (aq) + 3 SO42- (aq) + 3 H2 (g)
Uses of Praseodymium
Used As Alloys
Praseodymium has the potential to replace other lanthanides in the production of ‘Mischmetal,’ an alloy known for its pyrophoric properties. Praseodymium exhibits a strong affinity for other rare earth elements, leading to the formation of highly robust magnetic alloys. Praseodymium is commonly alloyed with magnesium for applications in the aircraft industry.
Used As Pigment
Praseodymium, an elemental constituent found in didymium glasses, plays a crucial role in their composition. The inclusion of praseodymium compounds in the composition of glasses and ceramics imparts a distinctive yellow hue to these materials.
Used As Magnets
Praseodymium finds application in high-intensity permanent magnets, which serve a pivotal function in the operation of electric motors and generators utilized in hybrid vehicles and wind turbines.
Used In Batteries
Praseodymium finds application in the context of nickel metal hydride (NiMH) rechargeable batteries utilized in hybrid automobiles. The cathode, which serves as the negative electrode in Nickel Metal Hydride (NiMH) batteries, consists of a blend of metal hydrides. These metal hydrides commonly include a combination of rare earth elements such as praseodymium, neodymium, lanthanum, and cerium.
Used In Optics
Praseodymium ions serve as a dopant in a multitude of applications within the realm of optics and photonics, encompassing optical amplifiers, fiber lasers, and various other cutting-edge technologies.
Used In Safety Gear
The application of praseodymium and neodymium oxides is evident in the production of protective eyewear for welders and glass blowers, aiming to provide defense against the harmful impacts of yellow flare and ultraviolet (UV) radiation on the eyes.
Used As Catalyst
Praseodymium oxide catalyzes the production of polyethylene, a highly prevalent plastic material employed in the manufacturing of various consumer products such as soda bottles, bubble wraps, food plastic wraps, sandwich bags, and milk cartons.
Health Effects of Praseodymium
- Praseodymium, like other rare earth metals, has a toxicity level between those of aluminum and tin.
- Ingestion of soluble praseodymium salts has been found to exhibit mild toxicity, whereas insoluble salts have been determined to be non-toxic. Skin and eye irritants are present in these substances.
- Praseodymium poses significant occupational hazards primarily attributable to the inhalation of vapors and gases in the workplace setting.
- Prolonged exposure to this substance has the potential to induce pulmonary embolisms, particularly in the context of extended duration. The accumulation of praseodymium in the human body has the potential to pose a risk to the liver.
Environmental Effects of Praseodymium
- Praseodymium is released into the environment through various sources, primarily by industries involved in petroleum production. It can also be introduced into the environment through the disposal of household equipment.
- Praseodymium has detrimental effects on cell membranes in aquatic animals, leading to adverse impacts on reproductive processes and the functioning of the nervous system.
- Over time, the element praseodymium tends to accumulate within both soil and water. Therefore, this process leads to heightened levels of praseodymium within the biological systems of humans, animals, and soil particles.
References
- https://www.rsc.org/periodic-table/element/59/praseodymium
- https://www.britannica.com/science/praseodymium
- https://byjus.com/chemistry/praseodymium/
- https://www.chemicool.com/elements/praseodymium.html
- David R. Lide, CRC Handbook of the Chemistry and Physics 86th Edition., Taylor and Francis., 2005, 4-32.
- https://www.lenntech.com/periodic/elements/pr.htm
- https://pubchem.ncbi.nlm.nih.gov/element/Praseodymium
- https://www.sciencedirect.com/topics/chemical-engineering/praseodymium
- Ferenc Szabadváry, Handbook of the Chemistry and Physics of the Rare Earths Vol. 11., Elsevier Science Publishers., 1998, p61.
- https://www.edge-techind.com/category/Praseodymium-97-1.html
- https://www.chemistrylearner.com/praseodymium.html
