Ionic compounds go through ionic polarization when the oppositely charged ions move in opposite directions because of the electric field. This gives the compound a net dipole moment. The ions move because the lattice is vibrating. The relative movement of the ions in the compound changes the center of the charges, which is affected by how symmetrical the movements are. When these centers do not correspond, polarization increases. Ion polarization is the name for this type of polarization.
What is Polarization of Ions?
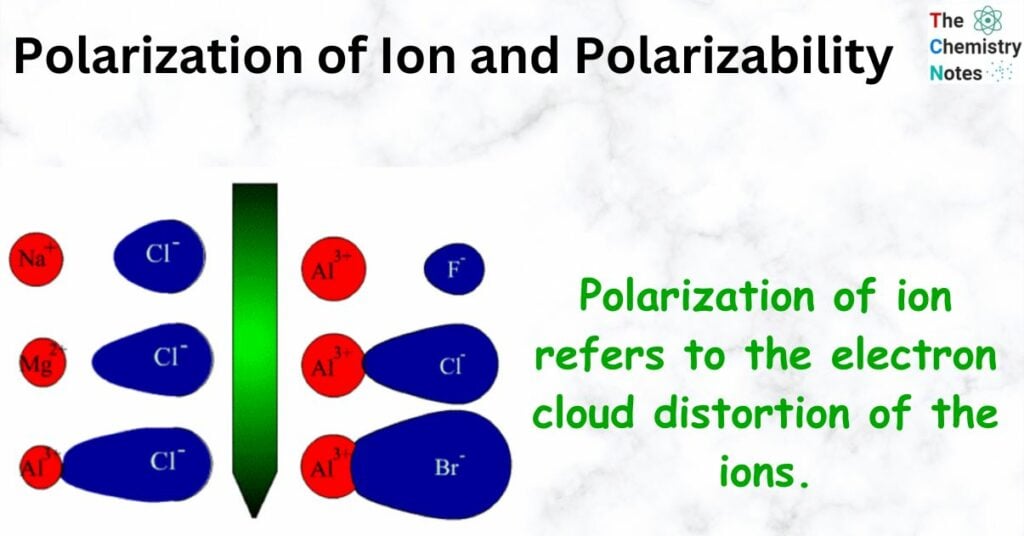
Polarization of ion refers to the electron cloud distortion of the ions.
- In the perfect ionic model, the ions would be perfect spheres, and the positive and negative ions would attract each other but not change each other. In reality, things are a little different because the positive ion will pull some of the electron cloud of the negative ion toward itself.
- If a cation has a lot of electric charge, it will have a strong electrostatic pull on an anion, which will change the shape of the electron cloud.
- If the anion has a big cloud of electrons, it is easy to change its shape.
- If the cloud of electrons is distorted, there will be a lot of electrons between the two ions, which will make the bond covalent.
- Fajan’s rules sum up all of these points.
These types of polarization of ions can be seen in the ionic compounds: NaCl, KCl
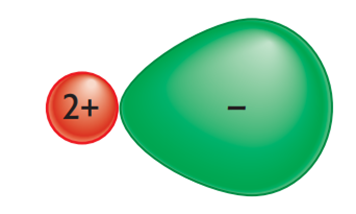
In our model of an ionic lattice, we have imagined that the ions are round. This does not always happen. In an ionic lattice, the positive charge on the cation can sometimes pull the electrons from the anion toward it. This causes the anion’s electron cloud to become distorted, and the anion is no longer round. This kind of distortion is called “ion polarization.” The power of a cation to attract electrons and change the shape of an anion is called its “polarizing power.”
Polarizing Power
Polarization power refers to a cation’s capacity to distort an anion.
Polarizing power of cation: Polarizing power is the ability of a cation to change the polarity of an anion. It is directly related to the charge density, which is directly related to the charge on cations and inversely related to the size of anions.
The power to polarize goes up as the size of the cation goes up. This means that smaller cations are very good at polarizing an anion. But the polarizing power goes up as the charge on the cation goes up.
Polarizability
Polarizability refers to the anion’s tendency to get polarized by the cation.
An anion’s tendency to become polarized: This is called its polarizability. It shows how easy it is for an anion to change shape when a cation is present. It has the same relationship as the anion’s size and negative charge. The bigger anions are more likely to change shape than the smaller ones. Also, it’s important to keep in mind that the anions with the most negative charge polarize easily.
Factors Affecting Polarization of Ion
The degree of polarization of an anion depends on the charge density of the cation the anion’s polarizability, or how easily it can be polarized.
An anion is more likely to be polarized if:
- The cation has a charge of 2+ or 3+ and is small.
- The anion is big and has a charge of 2– or 3–.
Small cation: The high polarizing power comes from the high concentration of positive charge in a small area. This shows why LiBr is more covalent than KBr (Li+ 90 pm vs. K+ 152 pm).
Large anion: The high polarizability comes from the larger size, where the outer electrons are held less tightly and can be twisted more easily by the cation. This explains why, out of the common halides, iodides are the most covalent in nature (I– 206 pm).
Large charges: As the charge on an ion goes up, the electrostatic pull of the cation on the outer electrons of the anion goes up, making it easier for covalent bonds to form.
Fajan’s Rule
The likelihood of ionic compounds to gain covalent character as a result of polarization of ion may be determined using Fajan’s principles. K. Fajan investigated the aspects that contributed to the covalent nature of ionic compounds and devised a series of guidelines known as Fajan’s rules.
- Even if a link in a molecule, such as X+Y–, is fully ionic, it will always have covalent qualities.
- When two ions with opposing charges (X+ and Y–) come into contact, the positively charged nucleus of the anion is repelled by the negatively charged cation. This causes the anion to become polarized, misshapen, or distorted.
- When the degree of polarization of ion is relatively low, an ionic bond occurs, but a covalent bond forms when the degree of polarization is high.
- The propensity of an anion to get polarized by a cation is known as its polarizability, whereas the capacity of a cation to distort an anion is known as its polarization power.
References
- Atkins, Peter; de Paula, Julio (2006). Atkins’ physical chemistry (8th ed.). Oxford: Oxford University Press. ISBN 978-0-19-870072-2.
- Barrow, Gordon M. (1988). Physical chemistry (5th ed.). New York: McGraw-Hill. ISBN 978-0-07-003905-6.
- Ashcroft, Neil W.; Mermin, N. David (1977). Solid state physics (27th repr. ed.). New York: Holt, Rinehart and Winston. ISBN 978-0-03-083993-1.
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/ Atomic_and_Molecular_Properties/Polarizability
- https://revisionscience.com/a2-level-level-revision/ chemistry-level-revision/bonding-and-structure/ nature-bonds
- https://www.savemyexams.co.uk/international-as/chemistry/edexcel/17/revision-notes/1- structure-bonding–introduction-to-organic-chemistry/1-6-ionic–metallic-bonding–structure/1-6-4-polarisation/
