Phosphorus is a chemical element with the atomic number 15 and the symbol P. Although phosphorus occurs in two primary forms, white phosphorus and red phosphorus, it is never found as a free element on Earth due to its extreme reactivity. Phosphorus is usually known as ‘phosphate’ in minerals.
It plays an important role in how the body uses carbohydrates and fats. It is also necessary for the body to make protein for the growth, maintenance and repairing of cells and tissues. Phosphorous is a multivalent nonmetal of the nitrogen group. It is found in nature in several allotropic forms, and is an essential element for the life of organisms.
Interesting Science Videos
History of Phosphorus
Phosphorus was firstly discovered by a german merchant and alchemist named Hennig Brand in the year 1669. Brand conducted an experiment with urine where he used around 50 buckets of urine which were allowed to stand until the time they putrefied and bred the worms. By first turning urine into a paste and then heating it with sand, Brand came up with a method for extracting elemental phosphorus from the combination. In a letter, he recounted his discovery and demonstration, as well as the element’s tendency to glow in the dark, It was named phosphorus mirabilis (a miraculous bearer of light).
James Burgess Readman of Edinburgh invented an electric furnace process for manufacturing the element from phosphate rock in the late 1800s, which is still in use.
Occurrence of phosphorus
The phosphorus content in the Earth’s crust is roughly one gram per kilogram. It does not exist in nature in its pure form, although it may be found in a number of minerals, most notably as phosphates, which consists of a phosphorus atom bonded to four oxygen atoms.
Inorganic phosphate rock, which includes some apatite, is currently the main commercial source of this element.The most heavily mined deposits are those of fluorapatite. Russia, the United States, Morocco, Tunisia, Togo, and Nauru are the primary mining countries. The global output is 153 million tons per year. There are questions about the sustainability of these phosphorus deposits.
White phosphorus is produced industrially by heating phosphate rock in a furnace with charcoal and silica. Phosphorus is produced as a vapour, which is subsequently collected beneath water. Red phosphorus is created by gradually heating white phosphorus in the absence of air to roughly 250°C.
Isotopes of Phosphorus
- Although phosphorous (15P) has 23 stable isotopes ranging from 25P to 47P, only 31P is stable; hence, phosphorus is classified as a monoisotopic element.
- The radioactive isotopes with the longest half-lives are 33P (25.34 days) and 32P (14.268 days). All others have half-lives of less than 2.5 minutes, with the majority being less than a second.
- With a half-life of less than 30 nanoseconds, 25P is the least stable.
Allotropes of Phosphorus
In nature, there are several allotropic forms of phosphorus. Phosphorus exists in at least ten allotropic forms, the most important of which are white phosphorus, black phosphorus, and red phosphorus.
- Diphosphorus (P2) is the gaseous form of phosphorus.
- White phosphorus (P4) has a tetrahedral structure.
- Red phosphorus contains more atoms connected together in a network than white phosphorus, making it far more stable.
- Violet phosphorus is created by heating and crystallizing red phosphorus in a certain way.
- Black phosphorus is the most stable form; the atoms are bound together in puckered sheets, similar to graphite.
Elemental Properties of Phosphorus
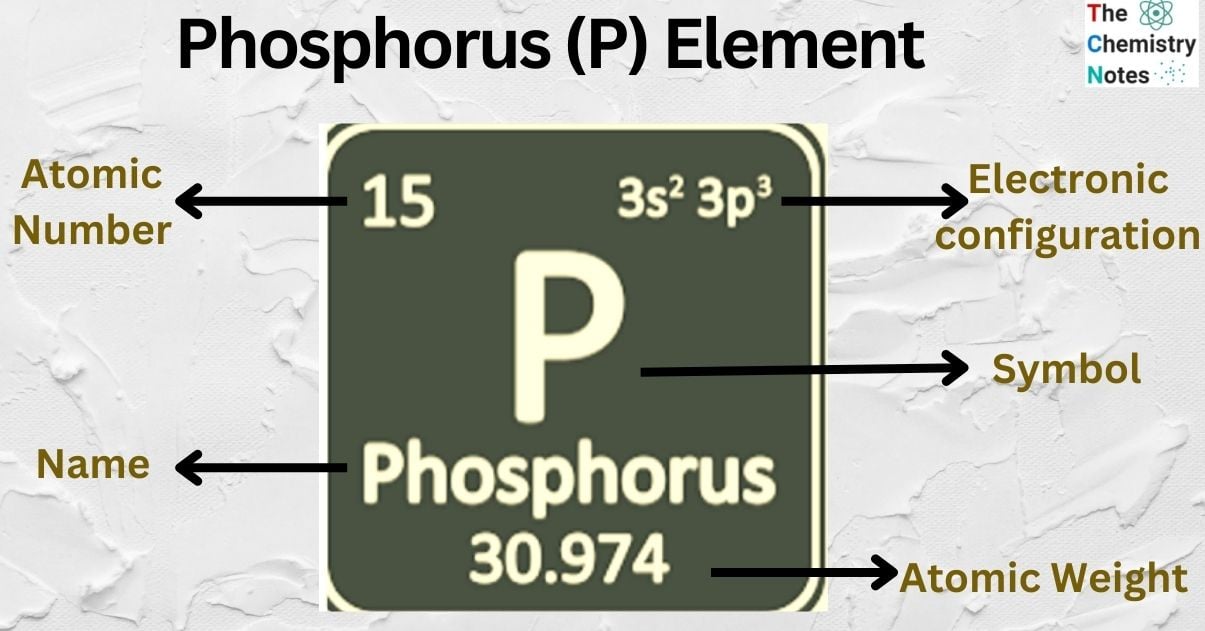
| Electronic Configuration | [Ne] 3s2 3p3 |
| Atomic Number | 15 |
| Atomic Weight | 30.974 |
| State at 20°C | Solid |
| Group, Period, and Block | 15, 3, p-block |
| Density | 1.823 (white) g.cm -3 at 20 °C |
| Empirical radius | 100 pm |
| Van der Waals radius | 180 pm |
| Electron shells | 2, 8, 5 |
| Electrons | 15 |
| Protons | 15 |
| Neutrons in most abundant isotope | 16 |
Physical Properties of Phosphorus
- Phosphorus is a multivalent nonmetal with a nitrogen group. It exists in nature in a variety of allotropic forms and is essential for organisms to survive.
- Phosphorus (white) has a melting point of 44.1 °C, a boiling temperature of 280°C
- Phosphorus comes in three allotropic forms: pale or white phosphorus, red phosphorus, and black phosphorus. The characteristics and reactivity of the chemical element phosphorus vary depending on the form.
White phosphorus is the industrial kind; it lights in the dark, is spontaneously combustible when exposed to air, and is a lethal toxin.It has a waxy and translucent appearance. Phosphorus has a boiling temperature of 240 degrees Celsius and a melting point of 44 degrees Celsius.
Red phosphorus may range in color from orange to purple,because of tiny differences in its chemical composition.Red phosphorus is typically seen on one side of a matchstick. Red phosphorus is produced by heating white phosphorus to 250 degrees Celsius. It does not dissolve in liquids.
Black phosphorus, is produced under high pressure, resembles graphite, and, like graphite, may conduct electricity.
| Color/physical appearance | white |
| Melting point/freezing point (white) | 44.15°C, 111.47°F, 317.3 K |
| Boiling point(white) | 280.5°C, 536.9°F, 553.7 K |
| Density(white) | 1.82 gram/cm3 at 20 °C (68 °F) |
| Malleability | No |
| Ductility | No |
| Electronegativity | 2.19 (Pauling Scale) 2.253 (Allen Scale) |
Chemical Properties of Phosphorus
- White phosphorus is the least stable, the most reactive, the most volatile, the least dense and the most toxic of the allotropes.
- White phosphorus slowly transforms into red phosphorus. Light and heat promote this change, therefore samples of white phosphorus nearly always contain some red phosphorus and hence appear yellow.
- When exposed to oxygen, white phosphorus emits a mild green and blue light in the dark.
- When exposed to air, it becomes extremely flammable and pyrophoric (self-igniting).
- The odour of combustion of this form has a characteristic garlic smell.
- White phosphorus is water insoluble but soluble in carbon disulfide.
Chemical Reaction of Phosphorus
Reaction of phosphorus with air
When exposed to wet air, white phosphorus shines in the dark, a phenomenon known as chemiluminescence. White phosphorus should be handled with extreme caution. At roughly room temperature, it spontaneously burns in air to generate “phosphorus pentoxide” – really tetraphosphorus decaoxide, P4O10.
P4 (s) + 5O2 (g) → P4O10 (s)
Reaction of phosphorus with the halogens
At normal temperature, white phosphorus, P4, interacts strongly with all halogens to generate phosphorus trihalides. As a result, it combines with fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2) to generate PF3, PCl3, PBr3, and PI3.
P4 (s) + 6F2 (g) → 4PF3 (g)
P4 (s) + 6Cl2 (g) → 4PCl3 (l)
P4 (s) + 6Br2 (g) → 4PBr3 (l)
P4 (s) + 6I2 (g) → 4PI3 (g)
In carbon disulphide (CS2), white phosphorus, P4, combines with iodine, I2, to generate phosphorus(II) iodide, P2I4. At 180°C, the identical chemical is generated via the interaction of red phosphorus and iodine, I2.
P4 (s) + 4I2 (g) → 2P2I4 (g)
Reaction of phosphorus with acids
Phosphorus does not react with non-oxidizing dilute acids.
Uses of Phosphorus
- The comparatively stable red phosphorus is used to create safety matches, tracer bullets, incendiary devices, insecticides, pyrotechnic devices, and a variety of other items.
- The phosphorus compound is mostly used in fertilizers. Ammonium phosphate is used to make phosphate ores. Before being turned into ammonium phosphate, the ores are first transformed into phosphoric acid.
- Phosphate is an important component of fertilizers and contributes to excellent crop yields. It is essential to the whole agricultural business, and there is no easy alternative.
- Bone ash (calcium phosphate) is used to make chinaware and to make monocalcium phosphate for baking powder.
- Trisodium phosphate is used as a cleaner, water softener, and scale/corrosion inhibitor.
Health Effects of Phosphorus
- Phosphates are the most frequent form of phosphorus in the environment. Phosphates are crucial elements in the human body because they are components of DNA and help in energy supply. Phosphates are also often found in plants.
- In its pure form, phosphorus is white. White phosphorus is the most harmful phosphorus source we know of. When white phosphorus develops naturally, it can be dangerous to human health. White phosphorus is very poisonous and can be lethal in many circumstances.
- Humans have significantly altered the natural phosphate supply by adding phosphate-rich manure to the soil and using phosphate-containing detergents. Phosphates are also found in a variety of foods, including cheese, sausages, and hams. Too much phosphate can harm your health by causing renal damage and osteoporosis. Phosphate shortages are also possible. Phosphate deficiency can be harmful to your health.
- In most cases, persons who died from white phosphorus exposure had mistakenly consumed rat poison. People who have been exposed to white phosphorus frequently feel nausea, stomach pains, and lethargy before dying.
- Skin burns can be caused by white phosphorus. While burning, white phosphorus can harm the liver, heart, and kidneys.
Environmental Effects of Phosphorus
Concerns regarding the environmental implications of the phosphate mining industries have grown in the last decade. The majority of the consequences are manifested as changes in local hydrology, water contamination, water consumption, air pollution, and human danger.
Phosphate mining activities are projected to raise the concentrations and loads of several dissolved and suspended hazardous metals and radioactive elements in the environment, including some that are particularly dangerous for water quality, air pollution, and human health.
White phosphorus does not react fast with other particles in water, therefore it accumulates in the bodies of aquatic species. Phosphorus will persist in soil for several days before being transformed into less hazardous compounds. However, phosphorus may persist in deep soils at the bottoms of rivers and lakes for a thousand years or more.
Fun Facts About Phosphorus
- Phosphorus is described as the “Devil’s Element” by some texts due to its eerie luminescence, tendency to burst into flame, and is the 13th known element.
- The Earth may be nearing “peak phosphorus,” after which the element may become increasingly difficult to extract.
- Phosphorus is required by all living creatures. The typical adult contains roughly 750 grams of phosphorus. It can be present in DNA, bones, and as an ion required for muscle contraction and nerve conduction in the human body.
- According to a 2013 research published in the journal Proceedings of the National Academy of Sciences, meteorites may have introduced phosphorous to Earth.
- Hennig Brand’s method for extracting phosphorus from urine was maintained a closely guarded secret. He decided to market his method to other alchemists. When it was sold to the French Academy of Sciences in 1737, this technique became more generally recognized.
Watch out the video with interesting experiments with the phosphorus element. DO NOT TRY AT HOME.
References
- Mary Elvira Weeks, The discovery of the elements. XXI. Supplementary note on the discovery of phosphorus J. Chem. Educ., 1933, 10 (5), p 302.
- https://study.com/learn/lesson/nitrogen-phosphorus-physical-chemical-properties.html
- https://www.chemicool.com/elements/phosphorus.html
- Mary Elvira Weeks, The discovery of the elements. II. Elements known to the alchemists J. Chem. Educ., 1932, 9 (1), p 11.
- https://byjus.com/chemistry/phosphorus/
- https://pubchem.ncbi.nlm.nih.gov/element/Phosphorus#section=Information-Sources
- https://www.livescience.com/28932-phosphorus.html
- https://sciencenotes.org/phosphorus-facts/
- Emsley, John (2000). The Shocking history of Phosphorus. A biography of the Devil’s Element. London: MacMillan. ISBN 0-333-76638-5.
- Parkes, G. D.; Mellor, J. W. (1939). Mellor’s Modern Inorganic Chemistry. Longman’s Green and Co.
- Shriver, Atkins. Inorganic Chemistry, Fifth Edition. W. H. Freeman and Company, New York; 2010; p. 379.
- Podger, Hugh (2002). Albright & Wilson. The Last 50 years. Studley: Brewin Books. ISBN 1-85858-223-7.
- https://www.lenntech.com/periodic/elements/p.htm

