Phase transfer catalysis (PTC), which allows reactions to be carried out on a wide scale and under mild conditions, is a practical and helpful technique for the synthesis of carbohydrates. Starks used the term phase-transfer catalysis in 1971 to describe the crucial role of tetraalkylammonium or phosphonium salts (QX) in reactions between two compounds in separate immiscible phases.
Phase transfer catalysis is a significant amplified extraction-reaction technique that is used in a wide range of chemical synthesis applications. Through the use of a heterogeneous transfer catalyst that can transport a reactant species between two immiscible phases, this approach enables reactions that are otherwise not possible via standard synthesis procedures. These biphasic circumstances allow for novel synthesis methods, higher yields, and faster reactions, as well as the separation of specific species.
What is phase transfer catalysis?
Phase transfer catalysis is used to perform reactions in two or more phases involving two or more reagents when the reaction becomes difficult due to the reactants’ difficulty coming together. Historically, phase transfer catalysts were mostly limited to group 15 onium compounds, specifically ammonium and phosphonium salts. Chiral ammonium salts in particular are shown to be quite effective at promoting asymmetric Phase transfer catalysis.
A “phase-transfer agent” is used to move one of the reagents to a position where it can easily and quickly react with another. The transferred species must also be in a highly active condition when transferred; otherwise, substantial amounts of phase-transfer agent will be required. This activation function, together with the transfer function, enables phase-transfer catalysis with only a catalytic amount of phase-transfer agent or catalyst.
Phase transfer catalysts
A phase-transfer catalyst facilitates the reaction between common organic substances soluble in organic solvents and substances soluble in water, like inorganic salts, in a heterogeneous system. The reaction can be carried out in a biphasic system comprising an affordable nonpolar aprotic solvent and water, without the need for high-polar solvents such as DMSO or DMF. A phase-transfer catalyst is soluble in both solvents and transports anions of inorganic salts into organic solvents before returning them to the water phase. Reactions often develop under mild conditions with simple work-up approaches.
A phase-transfer catalyst (PTC) is a catalyst that allows a reactant to migrate from one phase to another where the reaction happens. One unique type of heterogeneous catalysis is phase-transfer catalysis. In the absence of a phase-transfer catalyst, ionic reactants are frequently soluble in an aqueous phase but insoluble in an organic phase. The catalyst acts as a detergent, allowing the salts to be dissolved into the organic phase. The addition of a phase-transfer catalyst accelerates the process, which is referred to as phase-transfer catalysis.
Quaternary ‘Onium’ salts like ammonium, phosphonium, Crown ether, and cryptands are used as phase transfer catalysts. Some commonly used phase transfer catalyst involves:
l) Aliquat 336: Methyl trioctylammonium chloride
2) Benzyl trimethylammonium chloride or bromide (TNIBA)
3) Benzyl triethylammonium chloride
4) Cetyl trimethylammonium chloride or bromide (CTMAB)
Mechanism of phase transfer catalysis
The mechanism of phase-transfer catalysis can be separated into two groups, with different active paths but comparable ultimate effects. In other words, the anion is allowed to enter the organic phase and is free to react with the substrate in both types.
Quaternary phosphonium or ammonium salts
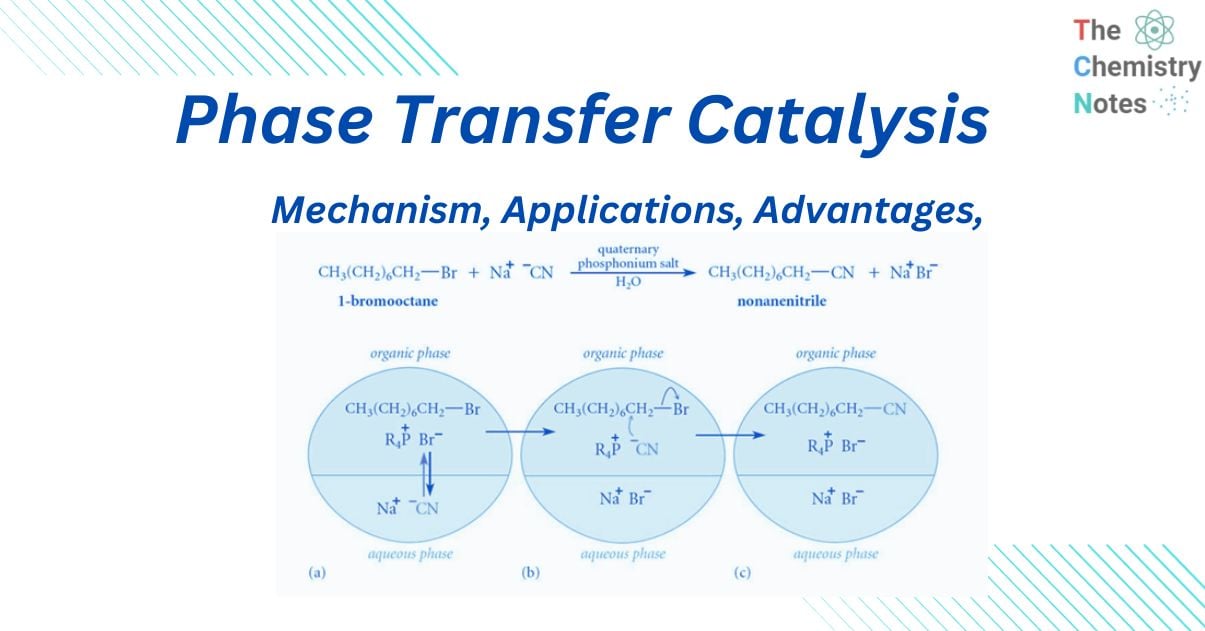
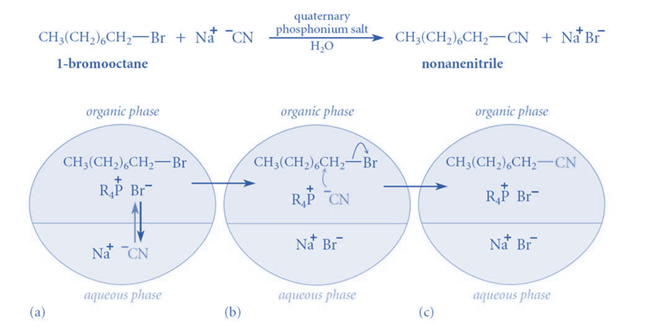
This mechanism can be appreciated by considering the reaction between sodium cyanide and 1-chloroethane, in which no substituted product is generated even after weeks of heating (with constant stirring). Even when a very tiny amount of a suitable “quaternary ammonium salt” is added, a significant amount of substituted product is created in less than two hours.
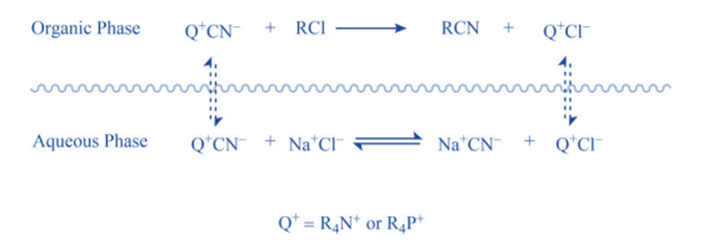
When “quaternary ammonium salt” is absent, there is no reaction because CN- ions cannot pass from the aqueous phase to the organic phase in sufficient concentration without leaving behind Na+ ions because this would interrupt the electrical neutrality of both phases, and Na+ ions have no motivation to go into organic phase because they are highly hydrated in the aqueous medium. When “quaternary ammonium or phosphonium salts” are introduced, quaternary ammonium (R4N+) and quaternary phosphonium ions (R4P+) are formed, which can pass over the interface between two phases.
This is due to the fact that the ‘R’ groups in R4N+ and R4P+ are relatively bulky, resulting in poor aqueous phase solvation. As a result, when they traverse the phases’ interface, they bring CN– ions with them to maintain the electrical neutrality of both phases. When the CN– ions reach a sufficient concentration, they begin to react with RCI, resulting in RCN and halide anions (CI–).
Crown ether and cryptands
Crown ether can selectively bind alkali and alkaline earth ions, generating complexes that are soluble in non-polar solvents due to their lipophilic nature, acting as carriers of the charged species. As a result, they’ve seen a lot of applications in phase transfer catalysis and ion extraction. For example, when KCN salt is combined with dicyclohexano-18-crown-6, a new salt is formed with the same anion but a considerably larger cation, as shown below.

Furthermore, because cryptand salt is quite soluble in many solvents, including organic types, we can introduce this salt directly into the organic phase without using an aqueous medium.
Factors Affecting Phase Transfer Catalysis
- Interfacial space
The quantity of interfacial space available governs the overall opportunity for any transfer in a two-phase system. As a result, dispersing one phase as small droplets in the second phase is crucial.
- Nature of anion
Large weakly-hydrated or organic anions such as perchlorate, iodide, and phenolate transfer well, but small hydrated anions such as fluorides or hydroxide move poorly.
- Bulkiness of catalyst
The bulkiness of the catalyst lowers transfer rates by lowering the maximum achievable concentration at the interface.
- Agitation
Only the transfer rate is affected by agitation. The agitation speed should be high enough such that it does not affect the overall reaction rate.
- H2O concentration
Having a saturated aqueous solution with anions that are difficult to transfer shifts the equilibrium towards the catalyst substrate complex. Furthermore, using less water reduces ion hydration.
- Organic solvent
Organic solvents Can have a significant impact on the intrinsic reaction rate. It affects the interfacial tension, which affects the transfer rate. Because it dissolves most quaternary salts and is hydrophobic, CH2C12 is widely used. Non-polar solvents like toluene are commonly employed for asymmetric PTC because they maximize interactions between the two counterions.
- Temperature
Temperature can have a significant impact on the intrinsic reaction rate. Most quaternary ammonium compounds degrade at higher temperatures, such as 50-70 C in KOH-containing solutions.
Advantages of phase transfer catalysis
- There is no need for vigorous conditions, and the reaction is quick.
- No expensive aprotic solvents are required.
- High temperatures are not required; the reaction normally occurs at low temperatures.
- Anhydrous conditions are unnecessary because water is used as one of the phases.
- It reduces the reliance on organic solvents.
- excellent scalability and inherent compatibility with moisture
- It increased reactivity, which allows for shorter reaction times and higher yields.
- Phase transfer catalysis has the ability to substitute costly and inconvenient reagents (such as LDA) for simple aqueous bases (such as KOH).
- Enantioselective variations are possible.
Application of phase transfer catalysis
- Phase transfer catalysis is frequently employed in commercial production industries.
- It is utilized to manufacture insecticides by alkylation of phosphothioates.
- Chiral quaternary ammonium salts are used as phase transfer catalysts for asymmetric alkylations.
- Phase transfer catalysis is not restricted to systems containing hydrophobic and hydrophilic reactants and is occasionally used in liquid-solid and liquid-gas processes.
- Phase-transfer catalysts are very useful in green chemistry because they allow the use of water, reducing the need for organic solvents.
- The synthesis of phenylacetic acid, an intermediate in the perfumery industry, is an example of a perfumery and fragrance industry.
- In the area of pharmaceuticals, Phase transfer catalysis is used in the synthesis process of numerous medications such as dicyclonine, phenoperidine, oxaladine, ritaline, and others.
- Polymeric-bonded PTC for the detection of cyanide, iodide, nitrite, sulfide, and thiocyanate enabled simple layer separation and PTC-free insertion of the sample into the chromatograph.
- PTC has enabled the use of less expensive and more readily available alternative raw materials such as potassium carbonate and aqueous NaOH solution in nucleophilic substitution reactions and reactions in the presence of bases involving the deprotonation of moderately and weakly acidic organic compounds, thereby eliminating the need for severe anhydrous conditions. Metal hydrides and organometallic reagents are both expensive solvents and toxic bases.
References
- https://www.sciencedirect.com/topics/chemistry/phase-transfer-catalysis.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4617660/
- https://link.springer.com/chapter/10.1007/978-94-011-0687-0_1.
- https://macmillan.princeton.edu/wp-content/uploads/AM_phase-transfer-catalysis.pdf.
- https://www.jetir.org/papers/JETIR2204065.pdf
