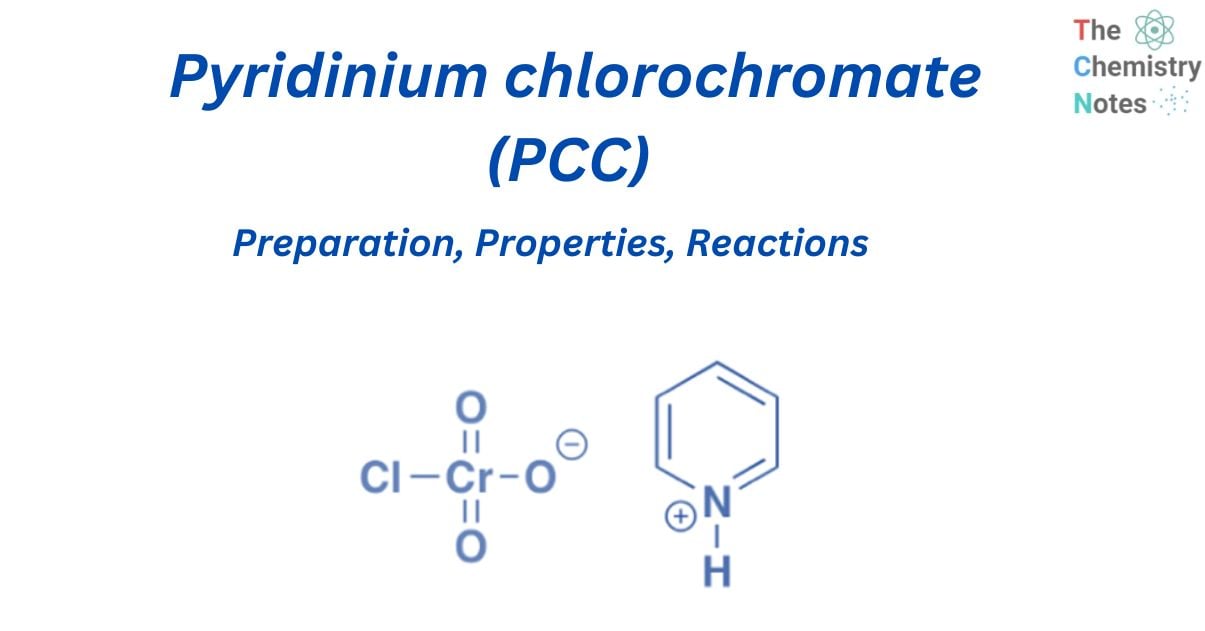
PCC (Pyridinium chlorochromate) is an oxidizing agent with the formula [C5H5NH]+[CrO3Cl]. It is a reagent in chemical synthesis that is mostly used to oxidize alcohols to generate carbonyls. Corey and associates first developed pyridinium chlorochromate, which is a stable orange solid and can be stored in the air.
Pyridinium chlorochromate is an extremely helpful reagent that can be used in slightly alkaline, anhydrous conditions to convert organoboranes into carbonyl compounds. In addition to converting (secondary alkyl)boranes to ketones.
Interesting Science Videos
What is PCC?
Pyridinium chlorochromate is an essential reagent in organic synthesis, particularly employed in the selective oxidation of alcohols to yield carbonyl compounds. It is a milder version of chromic acid. Pyridinium chlorochromate oxidizes alcohols one step higher on the oxidation ladder, from primary alcohols to aldehydes and secondary alcohols to ketones. Unlike chromic acid, PCC does not oxidize aldehydes to carboxylic acids.
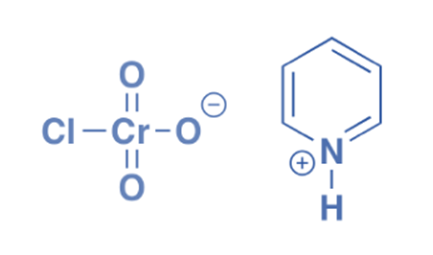
Preparation of PCC
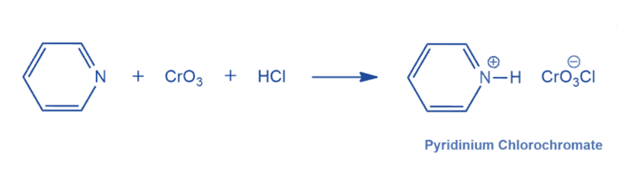
The reaction of pyridine, chromium trioxide, and hydrochloric acid produces Pyridinium chlorochromate. Chlorochromatic acid is made by dissolving chromium trioxide in aqueous hydrochloric acid. The addition of pyridine results in orange crystals of pyridinium chlorochromate.
Properties of PCC
- It is sensitive to moisture.
- It reacts with the easily oxidized material.
- It is incompatible with reducing agents, combustible material, metals, and strong reducing agents.
- In comparison to other regularly used chromium-containing oxidants such as Jons reagent and Colljn reagent, Pyridinium chlorochromate has strong oxidation ability, good selectivity, and the alkene bond of the substrate molecule is not damaged during the oxidation process.
- It is easy to handle.
Comparison between PCC and PDC ( Pyridinium dichromate)
PCC has similar features to PDC in that it is not extremely hygroscopic, is stable, commercially available, and can be stored. PCC is soluble in a wide range of organic solvents, the most common of which is dichloromethane at room temperature.
Both PDC and PCC can convert alcohols into aldehydes and ketones at room temperature, particularly in dichloromethane. PDC is less acidic than PCC and so more suited for oxidation of acid-sensitive substrates.
Although Pyridinium chlorochromate is more acidic than PDC, acid-labile compounds can be oxidized in the presence of sodium acetate or other buffering agents such as carbonates.
Uses of PCC
Typically, pyridinium chlorochromate is employed to oxidize an allylic methylene group. Primary alcohol oxidation to aldehydes Pyridinium dichromate (PDC) or pyridinium chlorochromate in anhydrous environments such as dichloromethane oxidizes primary alcohols to aldehydes while avoiding overoxidation to carboxylic acids.
As an oxidizing agent, pyridinium chlorochromate is used to convert primary and secondary alcohols to aldehydes and ketones, respectively. It is used in the synthesis of cyclohexanone, (-)-pulegone, and lactones. It is crucial in the mono-oxidation of xylenes to tolualdehydes and arylhydroxyamines to nitroso compounds. It is also used to oxidize amino acids, L-cystine, aniline, cycloalkanols, vicinal and non-vicinal diols, and in the Babler oxidation reaction.
Reactions of PCC
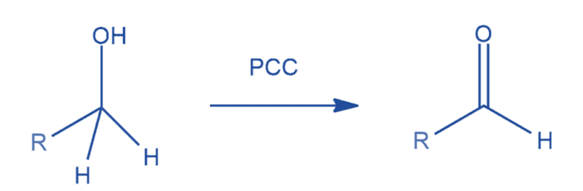
Oxidation of alcohol
It is a powerful reagent for converting primary and secondary alcohols to aldehydes and ketones, respectively. The reagent is more selective.
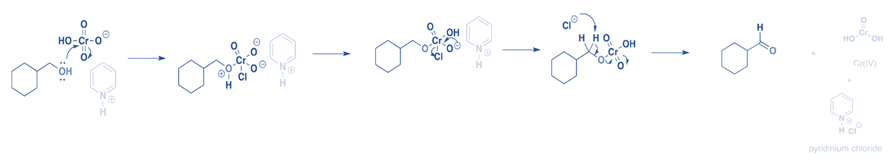
The first step is for oxygen to attack the chromium and create the Cr-O bond. Second, a proton from the (now positive) OH is transferred to one of the chromium’s oxygens, potentially via the pyridinium salt. A chloride ion is then displaced in a reaction similar to a 1,2 elimination reaction to generate a chromate ester.
When a base eliminates the proton on the carbon next to the oxygen, the C-O double bond is produced. The electrons from the C-H bond migrate to form the C-O bond, breaking the O-Cr connection in the process, and Cr(VI) becomes Cr(IV).
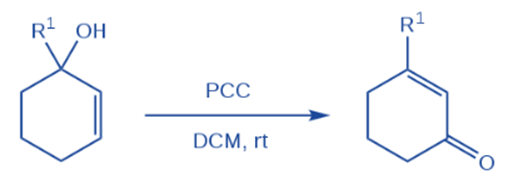
Babler oxidation
The Babler oxidation, also called the Babler-Dauben oxidation, is a chemical reaction that uses pyridinium chlorochromate to oxidize tertiary allylic alcohols to enones.
Oxidation of organoboranes
pyridinium chlorochromate is an extremely helpful reagent that can be used in slightly alkaline, anhydrous conditions to convert organoboranes into carbonyl compounds. In addition to converting (secondary alkyl)boranes to ketones.

References
- https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/pyridinium-chlorochromate
- https://pubmed.ncbi.nlm.nih.gov/10872568/
- https://www.organic-chemistry.org/chemicals/oxidations/pyridinium-chlorochromate-pcc.shtm
- https://www.chemistrylearner.com/pcc-reagent.html
