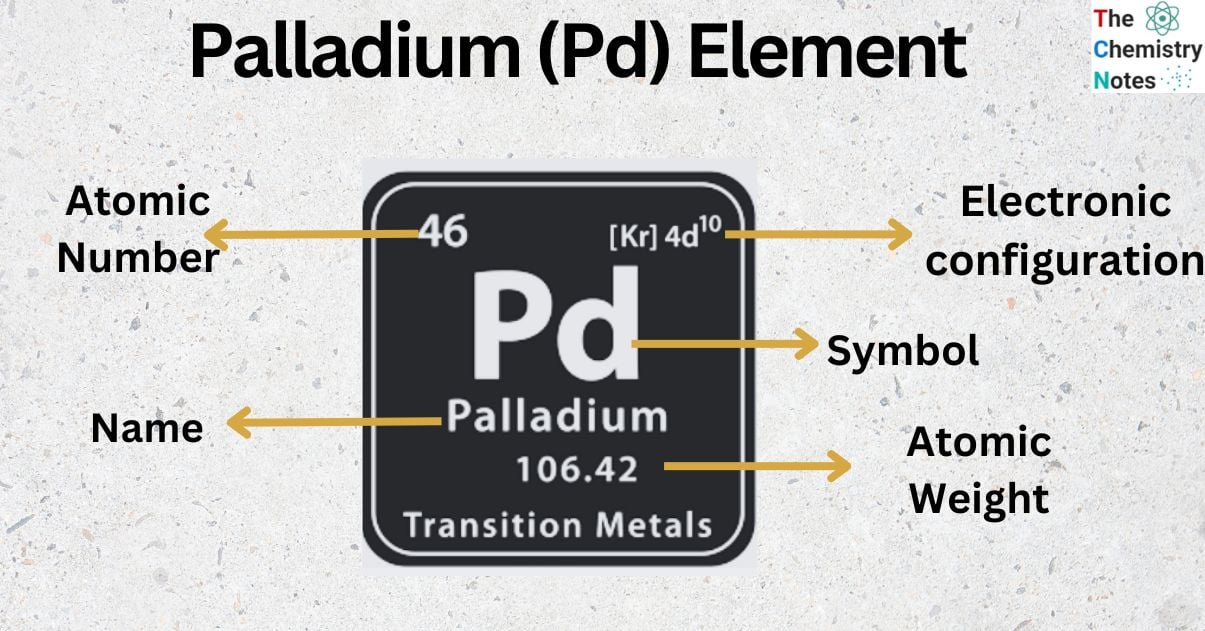
Palladium is a metallic element with the atomic number 46 and is represented by the symbol ‘Pd’ in the periodic table. It is classified as a transition metal and belongs to the d-block of group 10 of the periodic table. It is one of the rarest and most valuable metal that belongs to the platinum group of the periodic table. It has the lowest melting point and is least dense among the platinum group.
Palladium is a rare silvery-white and lustrous metal. It is one of the most expensive precious metals. Ore deposits of palladium are as rare as those of other platinum-group metals.
History of Palladium
- In the year 1803, an English chemist named William Hyde Wollaston discovered palladium while analyzing platinum ore samples extracted from South America.
- Wollaston named it after the asteroid Pallas, which was discovered earlier in 1802.
- Pallas is the name of the Greek god of wisdom.
Occurrence Of Palladium
- Palladium is among the most abundant of the platinum group metals. Its presence in the earth’s crust is about 0.015 ppm (parts per million). That makes it one of the rarest elements found in the crust.
- It usually occurs with platinum and other metals of the noble metal group.
- Palladium commonly occurs in two types of ore deposits: sulfide and platinum group elements (PGE). Sulfide deposits are the most common.
It can occur in ore minerals, natural compounds, or minerals with concentrated forms. Some of the most common palladium ore minerals are:
Palladium Sulfide (PdS): It serves as one of the key ore minerals for the metal and is frequently found with other PGEs and sulfide minerals, such as nickel sulfides. It is typically found in magmatic deposits and can also occur in hydrothermal veins.
Palladium Arsenide (PdAs2): The major ore mineral for palladium is palladium arsenide, which is frequently discovered alongside other PGEs like platinum, nickel, and copper. It is commonly found in magmatic deposits and hydrothermal veins.
Palladium bismuthide (PdBi): This is a rare palladium mineral that usually only occurs in trace amounts in hydrothermal deposits. It is usually associated with other bismuthide minerals and can occur along with other PGEs.
Palladium Antimonide (PdSb): This is another uncommon ore material that is occasionally encountered in hydrothermal deposits. It frequently co-occurs with other antimonide minerals and can be found in trace quantities in PGE-rich ore deposits.
Palladium Telluride (PdTe): This is a rare palladium ore mineral that is found in some magmatic and hydrothermal ore deposits. It is often associated with other telluride minerals, like gold tellurides.
Two of the most significant commercial sources of palladium are the Norilsk-Talnakh deposits in Siberia and the nickel-copper resources in the Sudbury Basin, Ontario.
Isotopes Of Palladium
Palladium has six naturally occurring stable isotopes: 102Pd, 104Pd, 105Pd, 106Pd 108Pd, and 110Pd.
Naturally occurring isotopes of Palladium
| Isotopes | Natural abundance (atom %) |
|---|---|
| 102Pd | 1.02 (1) |
| 104Pd | 11.14 (8) |
| 105Pd | 22.33 (8) |
| 106Pd | 27.33 (3) |
| 108Pd | 26.46 (9) |
| 110Pd | 11.72 (9) |
Elemental Properties of Palladium
| Electronic Configuration | [Kr] 4d10 |
| Atomic Number | 46 |
| Atomic Weight | 106.42 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 10, 5, d-block |
| Density | 12.023 g.cm -3 at 20 °C |
| Van der Waals radius | 163 pm |
| Electron shells | 2, 8, 18, 18, |
| Electrons | 46 |
| Protons | 46 |
| Neutrons in most abundant isotope | 60 |
Physical Properties of Palladium
- Palladium has an atomic number of 46 and is a silvery-white metal. It has a melting point of 1554.9 °C (2830.82 °F) and a boiling point of 2963 °C (5365 °F).
- It has a solid phase density of 12.023 gm/cm3 and a liquid or molten phase density of 10.38 gm/cm3.
- Palladium is malleable and ductile. This property allows it to be easily hit into sheets without cleavage and makes it possible to draw thin wires from it. The malleability of palladium is similar to that of gold.
- Palladium is not an excellent conductor of electricity,however it is able to conduct electricity due to the motion of electrons.
- Palladium demonstrates fascinating electromagnetic characteristics like high electrical conductivity and magnetic susceptibility.
- It is a relatively soft metal, with a hardness of 4.75 on the Mohs scale, which measures the hardness of minerals from 1 (the softest) to 10 (the hardest).
- When a surface is coated with finely divided palladium metal, hydrogen gas passes into the space between palladium atoms. It can absorb up to 900 times its weight in hydrogen gas.
- It has natural resistance to oxidation,It is as corrosion resistant as gold.
| Color/physical appearance | Lustrous, silvery-white |
| Melting point/freezing point | 1828.05 K (1554.9 °C, 2830.82 °F) |
| Boiling point | 3236 K (2963 °C, 5365 °F) |
| Density | 12.023 g cm-3 at 20° |
| Malleability | Yes |
| Ductility | Yes |
| Electronegativity | 2.2 (Pauling Scale) |
Chemical Properties of Palladium
- Due to its low reactivity, palladium does not easily tarnish or corrode in air or water. Although it can dissolve in aqua regia, a solution of nitrous and hydrochloric acids, it is immune to the majority of acids.
- Since palladium is the most reactive member of the platinum group element, it is “the least noble” of the noble metals.
- Palladium is unreactive with oxygen under normal conditions but will ignite a fire if ground into powder form.
- The metal is reactive with fluorine and chlorine when heated.
Chemical Reactions Of Palladium
- The Reaction of Palladium with Air
Palladium(II) oxide, PdO, is produced by heating palladium metal with oxygen.
2 Pd (s) + O2 (g) → PdO (s) [black]
- The Reaction of Palladium with Halogens
When palladium metal and fluorine gas, F2, are mixed under controlled conditions, it produces either palladium trifluoride or mixed-valence Pd (II, IV) salt [Pd]2+[PdF6]2-.
2 Pd (s) + 3 F2 (g) → [Pd] [PdF6] (s)
When palladium metal and chlorine, Cl2, react under the controlled condition of dichloride, palladium (II) chloride, PdCl2, is formed. Depending on the reaction conditions, one of two different forms of PdCl2 is formed.
Pd (s) + Cl2 (g) → PdCl2 (s)
When palladium metal and bromine, Br2, are mixed in a controlled atmosphere, the dibromide palladium (II) bromide, PdBr2, is formed.
Pd (s) + CBr2 (g) → PdBr2 (s) [red-black]
Uses Of Palladium
Palladium is one of the rare metals with unique properties, because of which it has a wide range of applications. Some of the uses of palladium are:
Used As Alloys: Palladium is a metal that easily forms alloys with other metals including platinum, gold, silver, and copper, which can change its properties and broaden its range of uses. some of the industries that use palladium alloy are:
Used In Dentistry: Palladium shows good solubility when mixed with other elements; it also has biocompatibility, corrosion resistance, and aesthetic properties, so it is often used as an alloy to make dental restorations and equipment.The metal is white like teeth and doesn’t become dull with time. It is a better option for dental procedures because of these qualities. Palladium alloys are used to make dental crowns, bridges, and dentures.
Used In Jewellery: Since palladium is naturally white and shiny it is used as a precious metal in jewelry, particularly in the production of engagement rings, wedding bands, and other jewelry items. Because of its inability to react with oxygen gas, palladium does not tarnish when exposed to air. Due to this characteristic, it is more precious than other jewelry metals like silver, which, with time, starts to seem dull. It is hypoallergenic, so it does not trigger any allergic reactions. Due to these characteristics, palladium is often used to make white gold (an alloy of gold and silvery metals).
Used As Catalyst: Palladium is frequently employed as a catalyst in different chemical reactions, especially in automobile catalytic converters that assist in lowering greenhouse gas emissions from vehicles. Palladium catalysts are also employed in petrochemical, pharmaceutical, and other industrial processes to speed up as well as enhance chemical reactions.
Used In Aerospace Industry:Palladium has a high melting point, so it is used, along with a few other precious metals like gold and silver, to make different parts of aircraft engines. It is naturally resistant to oxidation, so it is used to make airplane parts that need protection against weather and friction. It can be found along with other metals in heat exchangers, gasoline nozzles, and other places because it is strong, stable, and long-lasting.
Used In Electronics: The creation of multilayer ceramic capacitors, which are utilized in electronic gadgets like smartphones, tablets, and computers, is one of the many uses of palladium in the electronics sector. Additionally, it is also used in connectors, electrical contacts, and as a plating material on printed circuit boards.
Used For Water Treatment: Palladium is utilized in the manufacturing of screens for the purification of water and wastewater treatment, among other purification procedures. As a result of its ability to isolate hydrogen from other gases, it can be used to produce and purify hydrogen.
Health Effects of Palladium
- Consider all palladium compounds to be extremely poisonous and carcinogenic. If ingested, breathed, or absorbed via the skin, palladium chloride is poisonous and dangerous. These are from the experiments on animals.
- Several Pd salts have the potential to irritate the skin and eyes severely. Pd ions have a variety of harmful effects when used in vitro. minimal in vitro
- After being exposed to metallic Pd, cytotoxicity has been observed. It has been demonstrated that pd ions are strong skin sensitizers. Pd ions were found to be among the metals’ second-most common responding sensitizers in epidemiological research, right behind nickel.
- Pd allergy is frequently seen in people with documented nickel allergies. Nearly all people who have a Pd allergy also have nickel sensitivity. Additionally, there are several signs that suggest the possibility of respiratory sensitization (WHO).
Environmental Effects of Palladium
Palladium has little to no impact on our environment.
References
- https://www.rsc.org/periodic-table/element/46/palladium
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
- https://www.lenntech.com/periodic/elements/pd.htm
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1150–151. ISBN 978-0-08-037941-8.
- https://www.bbc.com/news/business-51171391

