The atmosphere of the Earth is composed of several strata. The ozone layer, also known as the ozonosphere, is a region of the upper atmosphere approximately 15 to 35 kilometers (9 to 22 miles) above the Earth’s surface that contains relatively high concentrations of ozone molecules (O3). 90 percent of the atmosphere’s ozone is found in the stratosphere, which extends from 10–18 km (6–11 miles) to approximately 50 km (about 30 miles) above the surface of the Earth. Due to the ozone layer’s absorption of solar radiation, the stratosphere atmospheric temperature increases.
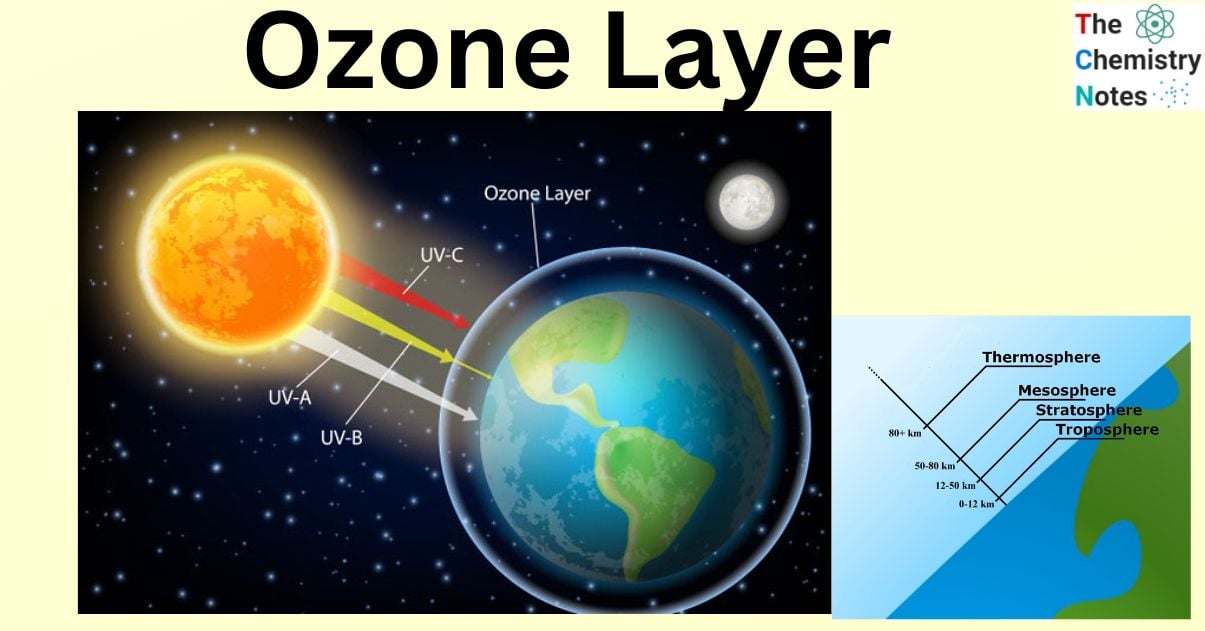
The ozone layer effectively prevents nearly all solar radiation with wavelengths shorter than 290 nm from reaching the Earth’s surface, including certain types of ultraviolet (UV) and other forms of radiation that could harm or destroy the majority of living organisms.
What is Ozone?
Ozone, (O3), is a triatomic allotrope of oxygen (a form of oxygen in which the molecule contains three atoms as opposed to two in its common form) and is primarily located in the stratosphere, where it shields us from the sun’s ultraviolet (UV) radiation.
Ozone is essential for existence on Earth, despite constituting only a minute fraction of the atmosphere. The ozone in the stratosphere, a layer of the atmosphere between 15 and 50 kilometers (10 and 31 miles) above us, functions as a shield to safeguard the Earth’s surface from the sun’s ultraviolet radiation. Without ozone, the Sun’s powerful UV rays would sterilize Earth’s surface. With the weakening of this shield, increased UV-B and UV-A radiation exposure at the surface would result in sunburns, skin cancer, and decreased crop yields.
What is Ozone Layer?
The ozone layer or ozone shield is a region of the stratosphere that absorbs the majority of ultraviolet radiation from the Sun. It contains a significant amount of ozone (O3).
The ozone layer, also known as the ozonosphere, is a region of the upper atmosphere roughly 15 to 35 kilometers (9 to 22 miles) above the Earth’s surface. The ozone layer effectively prevents nearly all solar radiation with wavelengths shorter than 290 nm from reaching the Earth’s surface, including certain types of ultraviolet (UV) and other forms of radiation that could harm or destroy the majority of living organisms.
Formation of Ozone
The production of ozone in the stratosphere is predominantly the result of high-energy solar photons disrupting the chemical bonds within oxygen molecules (O2). This process, known as photodissociation, releases solitary oxygen atoms, which combine with intact oxygen molecules to create ozone.
O2(g) → O(g) + O(g) (in presence of UV Radiation)
O(g) + O2(g) ↔ O3(g) (in presence of UV Radiation)
The formation of O3 is the result of UV radiation reacting with O2 or dioxygen molecules. The UV radiation separates the oxygen molecule into individual O atoms. These O atoms combine with oxygen molecules to create ozone (O3). The compound is thermodynamically unstable and tends to decompose into molecular oxygen. Therefore, there is a constant dynamic equilibrium between O3 molecule production and decomposition.
Approximately two billion years ago, rising atmospheric oxygen concentrations permitted ozone to accumulate in Earth’s atmosphere, a process that eventually led to the formation of the stratosphere. Scientists believe that the formation of the ozone layer played a significant role in the evolution of life on Earth by shielding life-threatening levels of UVB radiation (ultraviolet radiation with wavelengths between 315 and 280 nanometers) and thereby facilitating the migration of marine life to land.
Importance of Ozone Layer
At ground level, ozone is toxic, but in the upper atmosphere, the ozone layer performs a crucial function in protecting all life. UV rays from the sun have a negative impact on all forms of life. This layer soaks up the radiation and blocks it before it can reach Earth’s surface. The ozone layer is found in the uppermost stratosphere of Earth’s atmosphere. The contaminants that threaten Earth’s surface are filtered out by the layers that make up the lower atmosphere.
- There is a lot of O3 up in the stratosphere of Earth’s atmosphere. Therefore, this gaseous combination shields people and other living things from the sun’s damaging ultraviolet rays ( λ = 255 nm).
- Melanoma and other forms of skin cancer can develop in humans after prolonged exposure to high levels of UV light. Ozone layer filters the harmful rays reaching earth.
Ozone Layer Depletion
The term “ozone depletion” refers to the gradual lowering of the ozone layer in the high atmosphere.
Ozone depletion is induced by the production of chemical compounds containing gaseous chlorine or bromine from industry and other human activities, which causes a steady lowering of the Earth’s ozone layer. Ozone depletion is a serious environmental issue because it increases the amount of ultraviolet (UV) radiation that reaches the Earth’s surface, which raises the risk of skin cancer, eye cataracts, and genetic and immune system damage. The thinning is most noticeable in the polar areas, particularly over Antarctica.
Damaged regions of the ozone layer are commonly known as “ozone holes” however this is not the right term to label them. Damage to the ozone layer is more like a thin patch than a hole.
Cl + O3 → ClO + O2
ClO + O. → Cl + O2
O3 + O. → 2O2
Causes of Ozone Layer Depletion
- When chlorine and bromine atoms come into touch with ozone molecules in the stratosphere, they destroy them. Before it is evacuated from the stratosphere, one chlorine atom may damage over 100,000 ozone molecules. Ozone may be destroyed faster than it can be formed.
- Large volcanic eruptions, for example, can have an indirect influence on ozone levels. For example, the 1991 eruption of Mt. Pinatubo did not enhance stratospheric chlorine concentrations, but it did release a substantial number of small particles known as aerosols (different from consumer products also known as aerosols). These aerosols boost chlorine’s ability to deplete ozone. The stratospheric aerosols produce a surface on which CFC-based chlorine may deplete ozone. Volcanoes, on the other hand, have a short-lived effect.
- Free chlorine atoms and chlorine-containing gases, such as chlorine monoxide (ClO), may then break ozone molecules apart by removing one of the three oxygen atoms. Subsequent study indicated that bromine and some bromine-containing chemicals, such as bromine monoxide (BrO), were even more powerful at destroying ozone than chlorine and its reactive compounds.
- When some substances are exposed to high UV radiation in the stratosphere, they emit chlorine or bromine. These molecules, known as ozone-depleting substances, contribute to ozone depletion (ODS). Chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), carbon tetrachloride, and methyl chloroform are examples of ODS that emit chlorine. Halons and methyl bromide are examples of ODS that emit bromine. ODS are released near the Earth’s surface, but they are ultimately taken into the stratosphere, which can take two to five years.
- Concerns about the effects of ozone-depleting substances (ODS) on the stratospheric ozone layer led some nations, including the US, to prohibit the use of chlorofluorocarbons (CFCs) as aerosol propellants in the 1970s. Yet, global production of CFCs and other ODS continued to rise fast as new applications for these compounds were discovered in refrigeration, fire suppression, foam insulation, and other areas.
Not all chlorine and bromine sources deplete the ozone layer. Researchers discovered, for example, that chlorine from swimming pools, industrial facilities, sea salt, and volcanoes did not reach the stratosphere. ODS, on the other hand, are highly stable and do not disintegrate in rain. As a result, no natural mechanisms remove ODS from the lower atmosphere.
Ozone Depleting Substances
Ozone-depleting substances are man-made gases that destroy ozone once they get to the ozone layer. The ozone layer is in the upper atmosphere and cuts down on the amount of harmful UV radiation from the sun that reaches Earth. Ultraviolet radiation can be bad for both people and the environment. For example, it can cause skin cancer and cataracts, change how plants grow, and hurt the marine environment.
- chlorofluorocarbons (CFCs)
- hydrochlorofluorocarbons (HCFCs)
- hydrobromoflurocarbons (HBFCs)
- halons
- methyl bromide
- carbon tetrachloride
- methyl chloroform.
People have used them to refrigerants used in commercial, home, and car refrigerators and air conditioner, foam blowing agents, parts of electrical equipment, industrial solvents, cleaning solvents (even dry cleaning solvents), spray propellants, and fumigants.
Effects of Ozone Layer Depletion
- If the ozone layer disappears, people will be directly exposed to the sun’s harmful ultraviolet radiation. This could cause serious health problems for people, like skin diseases, cancer, sunburns, cataracts, early aging, and a weak immune system.
- Animals get skin and eye cancer from being directly exposed to ultraviolet radiation.
- Strong ultraviolet rays may stop plants from growing, flowering, and making food. The ultraviolet rays are also harmful to the forests.
- Harmful ultraviolet rays have a big effect on plankton. These are higher on the water’s food chain. If the planktons die, the other organisms in the food chain will also die.
- Temperature of the earth will increase leading to rise in sea level and flooding of low lying areas.
- Leaves of plants will show chlorosis (loss of chlorophyll and yellowing).
- UV radiation speeds up the rate at which water evaporates from a surface through stomata. So, it reduces the amount of water in the soil.
Solutions of Ozone Layer Depletion
- Lessen your consumption of ozone-depleting chemicals. Using CFCs in refrigerators and air conditioners should be avoided, and halon-based fire extinguishers should be replaced, among other things.
- Cars and trucks give off a lot of greenhouse gases, which heat up the planet and deplete the ozone layer. Because of this, people should drive their cars as little as possible.
- Use cleaning products that are good for the environment. Most cleaning products have chemicals that give off chlorine and bromine. These chemicals get into the air and hurt the ozone layer. To protect the environment, natural products should be used instead of these.
- The government should stop people from using nitrous oxide, which is bad for the ozone layer and should be banned. People should be told about the harmful effects of nitrous oxide and the things that give off the gas so that their use can be reduced.
Montreal Protocol
The Montreal Protocol, which was signed in 1987 and is officially called the Montreal Protocol on Substances That Deplete the Ozone Layer, is an international agreement to protect the stratospheric ozone layer by phasing out the production and use of substances that destroy ozone (ODS).
The Montreal Protocol has proven to be innovative and effective, and is the first international agreement to be ratified by all nations. Using this global participation, the Montreal Protocol has stimulated global investment in alternative technologies, many of which have been developed by U.S. companies, and put the ozone layer, which was in danger, on the path to recovery.
What is the Hole in The Ozone?
References
- Petrucci, Ralph H., William S. Harwood, and Geoff E. Herring. General Chemistry : Principles and Modern Applications. 9th ed. Upper Saddle River: Prentice Hall, 2006.
- Varotsos, Costas, Kirill Ya. Kondratyev. Atmospheric Ozone Variability: Implications for Climate Change, Human Health and Ecosystems. Chichester, UK: Praxis Publishing Ltd, 2000
- https://www.epa.gov/ozone-pollution-and-your-patients-health/what-ozone#:~:text=Stratospheric%20ozone%20is%20formed%20naturally,radiation%20reaching%20the%20Earth’s%20surface.
- https://byjus.com/biology/ozone-layer-depletion/#effects-of-ozone-layer-depletion
- https://environment.govt.nz/acts-and-regulations/acts/ozone-layer-protection-act-1996/ozone-depleting-substances/
- https://www.epa.gov/ozone-layer-protection/basic-ozone-layer-science#:~:text=II.-,Ozone%20Depletion,than%20it%20is%20naturally%20created.
- Parson, E. (2003). Protecting the Ozone Layer: Science and Strategy.
- https://education.nationalgeographic.org/resource/ozone-layer/

