With the IUPAC designation ethanedioic acid and a chemical formula of HOOC-COOH, oxalic acid is the most basic dicarboxylic acid. Several plants and vegetables possess this chemical component. A different term for it is oxalic acid. An odorless, crystalline dicarboxylic acid, oxalic acid (H2C2O4) is used in medicine. When dissolved in water, it creates a colorless solution. It is far more potent than acetic acid when it comes to acid strength.
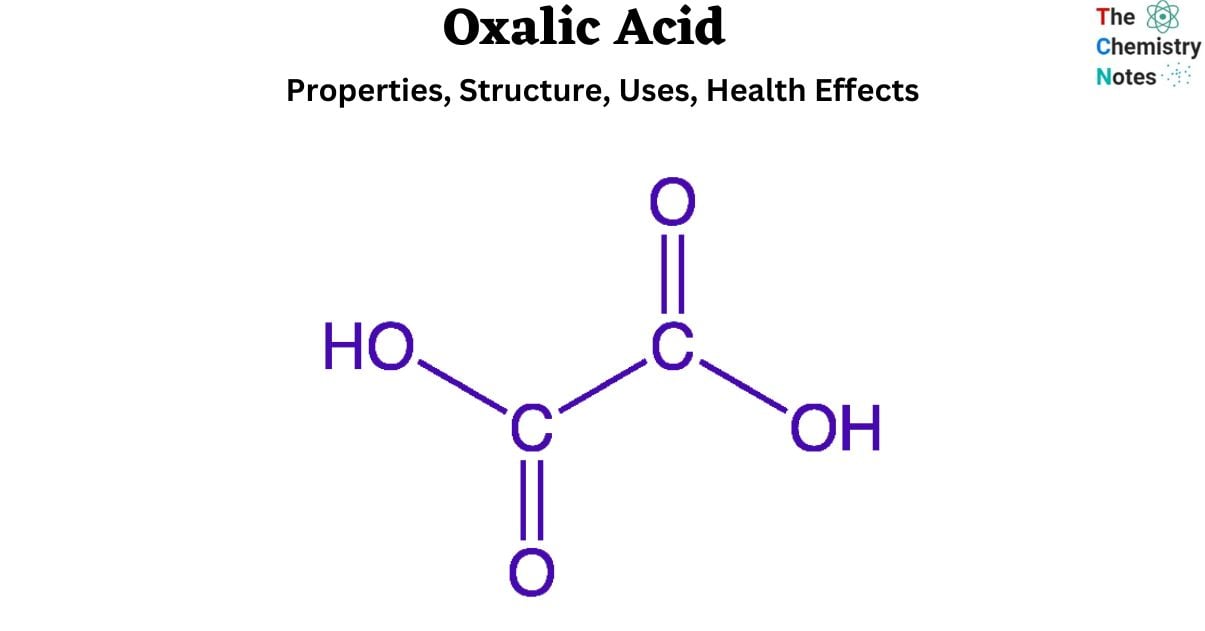
Oxalic acid is a reducing agent, and its conjugate base is oxalate. (C2O4)2- Oxalate functions as a chelating agent for metal cations. Typically, oxalic acid occurs in the form of a dihydrate with the formula H2C2O4.2H2O. The ingestion of oxalic acid, whether by skin contact or orally, is lethal.
Interesting Science Videos
History of Oxalic Acid
- It wasn’t until 1745 that plant-derived oxalic acid salts were produced. Hermann Boerhaave, a Dutch botanist and physician, produced oxalic acid from a sorrel salt.
- François Pierre Savary of Fribourg had effectively isolated oxalic acid from its salt in sorrel by 1773.
- Swedish chemists Carl Wilhelm Scheele and Torbern Olof Bergman synthesized oxalic acid by mixing sugar with strong nitric acid; Scheele named the resulting acid scker-Syra, which means sugar-acid or sweet acid.
- By 1784, it had been demonstrated that the sugar acid and oxalic acid acquired in nature are similar.
- In 1824, the German scientist Friedrich Wohler produced oxalic acid once more by reacting cyanogen with ammonia in an aqueous solution. This work may be regarded the first synthesis of a natural substance.
Physical Properties of Oxalic Acid
| Molecular formula | H2C2O4 |
| Molecular weight | 90.03 g/mol (anhydrous) 126.07 g/mol (dihydrate) |
| Appearance | Orthorhombic white crystals |
| Odor | Odorless |
| Boiling point | 1570 °C at 1.013hPa (anhydrous) |
| Melting point | 1010 °C sublimes at 1500 °C (dihydrate) 190 °C (anhydrous) |
| Flash point | 166 °C (331 °F, 439 K) |
| Density | 1.90 gm/cm3 (anhydrous) 1.653 gm/cm3 (dihydrate) |
| Solubility | Soluble in water, absolute alcohol |
Chemical Properties of Oxalic Acid
- Since oxalic acids are dibasic acids, they can give two H+ ions.
- Because it donates hydrogen, oxalic acid is an excellent reducing agent.
- Oxalates (C2O4)2- work well as chelating agents, creating chelate complexes with metal cations.
Oxalic Acid Formula
The chemical formula for oxalic acid is C2H2O4. It may be found in the cell sap of the plant species Oxalis and Rumex. Furthermore, it is a weak acid in an aqueous solution that can be slightly ionized. It also contains two acidic protons, which can be ionized. It is one of the most potent organic acids due to its characteristics and behavior. Oxalic acid is often referred to as diprotic acid.
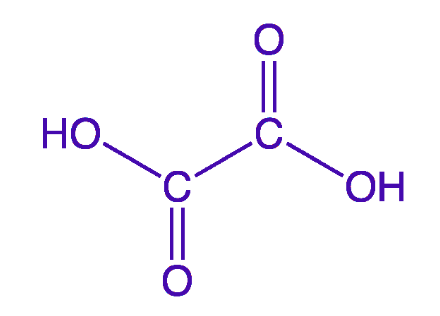
Structure of Oxalic Acid
Oxalic acid has two different polymorphs that occur in its anhydrous form. In the first polymorph of oxalic acid, hydrogen bonding occurs. At the intermolecular level, this hydrogen bonding results in a chain-like structure. The compound’s second polymorph is also capable of hydrogen bonding. However, hydrogen bonding imparts a sheet-like shape to the molecule at the intermolecular level in this example. This chemical is commonly employed in esterification processes, which have two significant features. The first attribute that makes oxalic acid suitable for esterification reactions is its acidic nature. The second and most essential aspect of oxalic acid is its hydrophilic nature, which means that it seeks water.
Equivalent Weight of Oxalic Acid
Hydrated oxalic acid has a molecular weight of 126 grams. Because its chemical formula is COOH-COOH, it is evident that oxalic acid is a dibasic acid capable of donating two H+ ions. As a result, the equivalent weight of oxalic acid may be calculated using the following formula:
Equivalent weight = (molecular weight)/(number of equivalent moles)Because 1 mole of oxalic acid may release 2 moles of H+ ions and neutralize 2 moles of OH– ions, the number of comparable moles is 2. To get the weight of oxalic acid equivalent, use the following formula:
Equivalent mass of oxalic acid = molecular mass of oxalic acid / 2
= 126 g / 2
= 63 grams.
Oxalic acid thus weighs 63 grams equivalently.
Preparation of Oxalic Acid
Oxalic acid is easily produced by concentrating nitric acid (HNO3) and oxidizing specific carbohydrates, such as sucrose. The carbon atoms are torn apart in pairings during oxidation, yielding oxalic acid.
- Step 1: Add 10 grams of cane sugar in a 250 ml conical flask.
- Step 2: Add 50 ml of concentrated nitric acid, then warm in the bain-marie of boiling water.
- Step 3: As the mixture heats, most of the sugar dissolves.
- Step 4: Take out the flask and place it on the wooden surface. After 15 minutes, transfer the heated solution onto an evaporating dish, followed by cleaning the flask with 10 ml of strong nitric acid and setting it in the evaporating dish.
- Step 5: The acid solution should be heated in a boiling water bath until it is reduced to around 10 ml, at which point it will begin to evaporate.
- Step 6: The ice-water combination helps the mixture to cool.
- Step 7: The crystallization of oxalic acid is quick after it cools.
- Step 8: Extract the product employing the Buchner funnel at the pump.
- Step 9: Press the product between the filter paper and place it in the air to dry it.
- Step 10: The resulting mixture yields approximately 3.5 g of oxalic acid.
Chemical Reaction of Oxalic Acid
There are two carboxyl groups in oxalic acid. Due to the two -COOH groups, it therefore doubles up on the majority of carboxylic acid group reactions.
- Oxalic acid is a stronger acid than acetic acid and rapidly forms a number of salts, including esters, acid halides, and amides. These are called mono- and di-derivatives of oxalic acid.
- Heat Treatment of Oxalic Acid: When heated to 150 degrees Celsius, oxalic acid decarboxylates, producing formic acid and carbon dioxide.
150 °C
HOOC - COOH → HCOOH + CO2- Sulfuric (H2SO4) reacts with oxalic acid to create carbon dioxide (CO2) and carbon monoxide (CO) while losing water (H2O).
conc. H2SO4 when heated Δ
HOOC - COOH → CO2 + CO + H2O- Oxalic acid is quickly oxidized to carbon dioxide and water in the presence of an acidified potassium permanganate solution.
KMnO4
HOOC - COOH + [O] → 2 CO2 + H2O
H2SO4Uses of Oxalic Acid
- Oxalic acid can form water-soluble complexes with ferrous ions present on metal surfaces. Because of its complex-forming feature, oxalic acid is employed as a cleaning agent to dissolve rust.
- Oxalic acids are employed in the process of coating the surface of aluminum, often known as aluminum anodizing. Oxalic acid is favored over sulfuric acid because its layers are thinner and smoother.
- Oxalic acid is employed as a bleaching agent in the bleaching of wood pulp. It is also used as a mordant to set colors on fabrics in the dye industry.
- Lanthanides, elements of the periodic table’s f-block, react with oxalic acid to generate hydrated lanthanide oxalate. These oxalates may then be heated to generate oxides of the elements; this procedure is important in separating pure lanthanides from a mixture of other elements.
- Because of its bleaching characteristics, oxalic acid is frequently utilized in teeth-whitening products.
Impacts of Oxalic Acid For Human Health
- Tissue is corroded by oxalic acid. When swallowed, oxalic acid causes calcium to be removed from the blood. Damage to the renal system is possible as a result of calcium oxalate being eliminated from the blood, which obstructs the renal tubules.
- It causes kidney damage and bloody urine when consumed.
- Inhalation can cause severe irritation and burns to the nose, throat, and respiratory system.
- Chronic exposure can cause upper respiratory tract irritation.
Video Reference
References
- https://byjus.com/chemistry/oxalic-acid/#:~:text=Oxalic%20acid%20is%20a%20dicarboxylic,strength%20greater%20than%20acetic%20acid.
- https://dept.harpercollege.edu/chemistry/msds/Oxalic%20acid%20dihydrate%20JTBaker.pdf
- https://www.britannica.com/science/oxalic-acid
- https://www.aakash.ac.in/important-concepts/chemistry/oxalic-acid
- https://www.vedantu.com/chemistry/oxalic-acid
- https://collegedunia.com/exams/oxalic-acid-chemistry-articleid-2581

