Organosilicon compounds are organometallic compounds that contain Si-C bonds. As the Si-C bond is extremely hard and stable, many organosilicon compounds containing Si-C have been synthesized. However, silicon has little tendency to form a bond with itself and does not form pπ-pπ double bonds. It can not replicate a wide range of organic compounds.
Preparation of organosilicon compounds
1. From Grignard reagent
The Grignard reagent reacts with tetrachlorosilane to give the organosilicon compounds. It is a useful method for preparing organosilicon compounds in the laboratory or on a small scale.
SiCl4 + CH3MgCl → CH3SiCl3 + MgCl2
CH3SiCl3 + CH3MgCl → (CH3)2SiCl2 + MgCl2
(CH3)2SiCl2 + CH3MgCl → (CH3)3SiCl + MgCl2
CH3)3SiCl + CH3MgCl → (CH3)4Si + MgCl2
2. Using organolithium compounds
The organolithium compound on reaction with tetrachlorosilane produces organosilicon compounds.
4 LiR + SiCl4 → SiR4+ 4 LiCl
3. Rachow process
It is the direct method of organosilicon compounds. In the Rachow process, the alkyl or aryl halides react with a fluidized bed of silicon at 280-300°C in the presence of a large amount of copper catalyst to produce chlorosilanes.
Si + 2CH3Cl → (CH3)2SiCl2
This is the main industrial method for making methyl and phenyl chlorosilanes which are of considerable commercial importance in the production of silicones. The separation of the methylchlorosilanes must be done with care because the properties of silicone polymers produced from these halides are critically dependent on the composition of the chloride mixture being hydrolyzed.
Silicones
Silicones are organo-silicon polymers. The organosilicon compounds containing O-Si-O linkage and Si-C bonds are known as silicones, and the polymers are known as siloxanes. Silicones have general formula (R2 SiO) X.
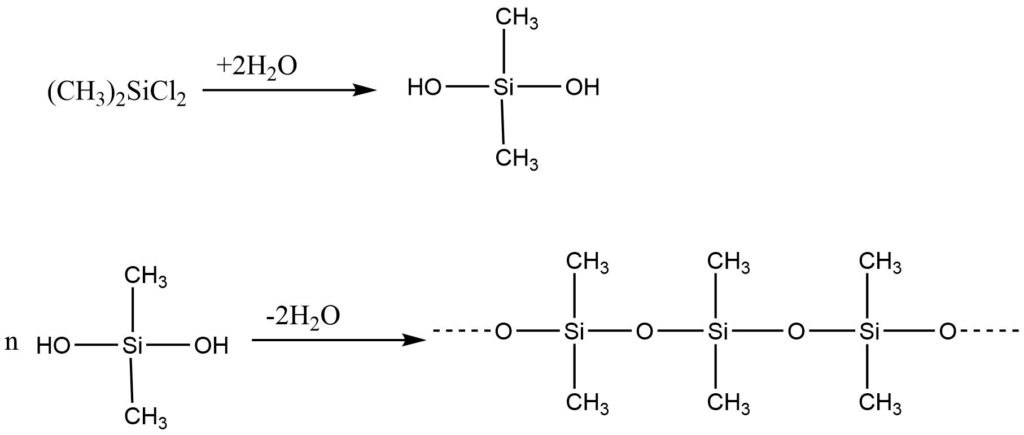
In the presence of a copper catalyst, silicon reacts with methyl chloride (CH3Cl) to form dimethyldichlorosilane [(CH3)2Si(Cl)2]. Hydrolysis of this compound produces silanol, which results from the replacement of chlorine atoms by the hydroxyl (OH) group. The resulting unstable compound, silanol ([CH3]2Si[OH]2), polymerizes via condensation, with single-unit molecules linking together to form poly-dimethylsiloxane.
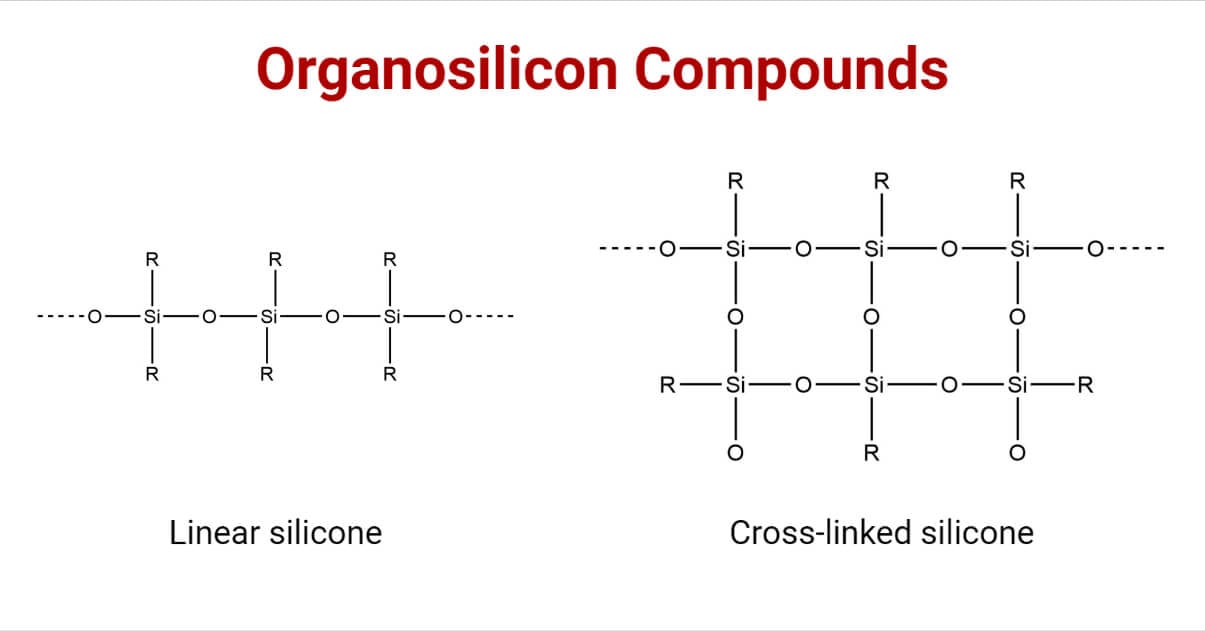
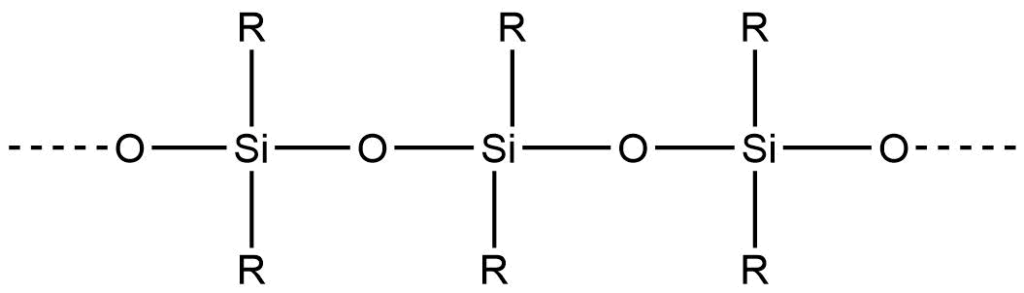
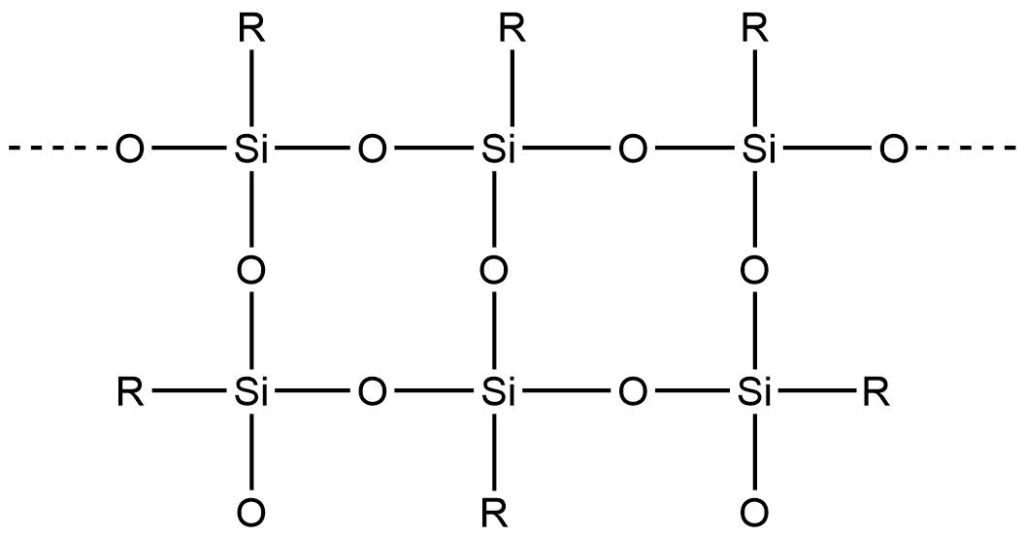
These silicones may be linear, cyclic, or cross-linked.
Properties of silicon
Silicon polymers have an inorganic -(Si-O)- backbone with high surface energy.
- They have low thermal and electrical conductivity.
- They have high gas permeability.
- Most silicones are very flexible due to low rotation barriers.
- They are not inflammable and are excellent electrical insulators.
- They have low toxicity.
Preparation of silicones
The alkyl or aryl-substituted chlorosilanes act as the starting material for the preparation of silicones.
1. On hydrolysis, dialkyldichlorosilane gives the silane diol, which undergoes intermolecular condensation producing linear or straight-chain polymers.
2. Hydrolysis of dialkyldichloro silane under carefully controlled conditions can produce various products. Under controlled conditions, diol undergoes rapid condensation to give a mixture of linear and cyclic polysiloxane containing three, four, or six Si atoms.
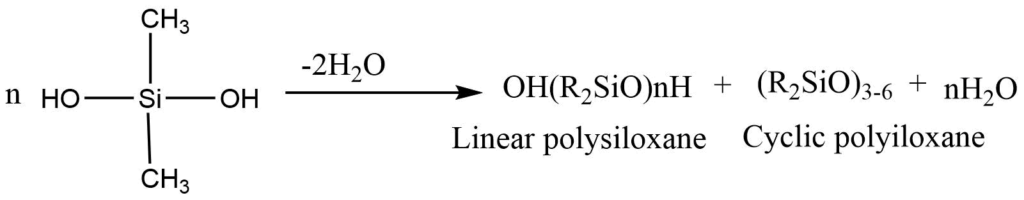
3. The hydrolysis of trimethylmonochlorosilane yields trimethyl silanol, a volatile liquid. Which condenses to give hexamethyl disiloxane. Since this compound has no OH group, it cannot polymerize further.
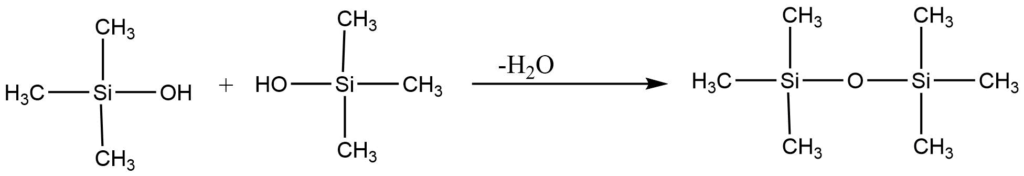
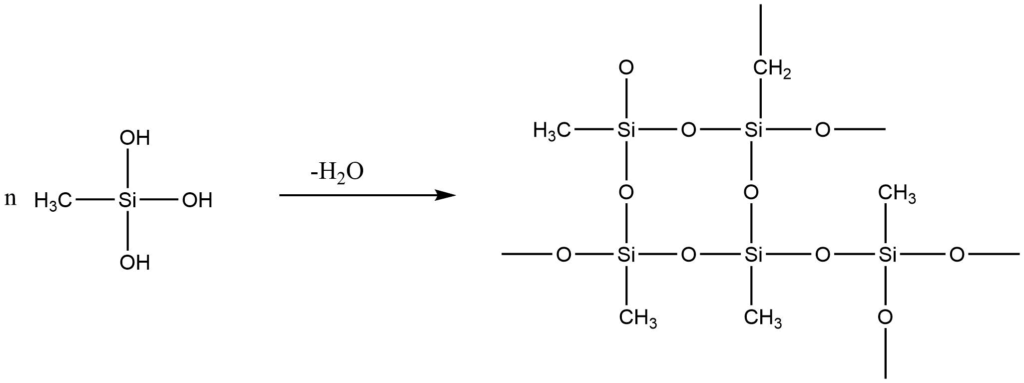
4. Alkyltrichlorosilane, on hydrolysis, produces silane triol, which on condensation, yields very complex cross-linked polymers.
CH3 SiCl3 + 3H2O → CH3Si(OH)3 + 3H2O
Application of silicones
1. Silicons are water-resistant and useful for making waterproof industrial materials. The polar Si-O bonds orient themselves near the surface, and the organic group presents a water-repelling exterior.
2. They are used in lubricants and for making high-temperature resistance paints.
3. These compounds are also important components of resins. The structure and properties of silicone give the resin a much more stable structure and make it a more effective binder. Silicon resins are used in the insulation of electrical equipment and electronic circuits, as well as for the encapsulation of components such as resisters and integrated circuits.
6. Silicones are excellent materials for improving the efficiency, durability, and performance of solar panels and photovoltaic devices.
7. The silicones with a smaller degree of cross-linking are rubber. They are used for making gaskets, valve seals, etc., since it is highly flexible.
8. Breast implants are made from highly viscous silicones enclosed in biologically compatible pouches. They are physiologically inert and thus suitable for such applications.
References
- https://byjus.com/jee/silicones/
- https://www.britannica.com/science/silicone
- https://www.nature.com/articles/204512b0
- https://polymerdatabase.com/polymer%20classes/Silicone%20type.html
- https://www.chemicalsafetyfacts.org/silicones-post/
- https://www.products.pcc.eu/en/blog/what-is-silicone/
- https://www.lkouniv.ac.in/site/writereaddata/siteContent/202004061919579825abhinav_Organo_Silicon_Compounds.pdf
- S. Pimplapure, R. Jain, A. Sahai, U. Soni, Inorganic Polymer Chemistry. Pragati Prakashan, Meerut, 2012.
