Organic halogen compounds are a broad family of natural and manmade substances containing one or more halogens (fluorine, chlorine, bromine, or iodine) in combination with carbon and other elements.
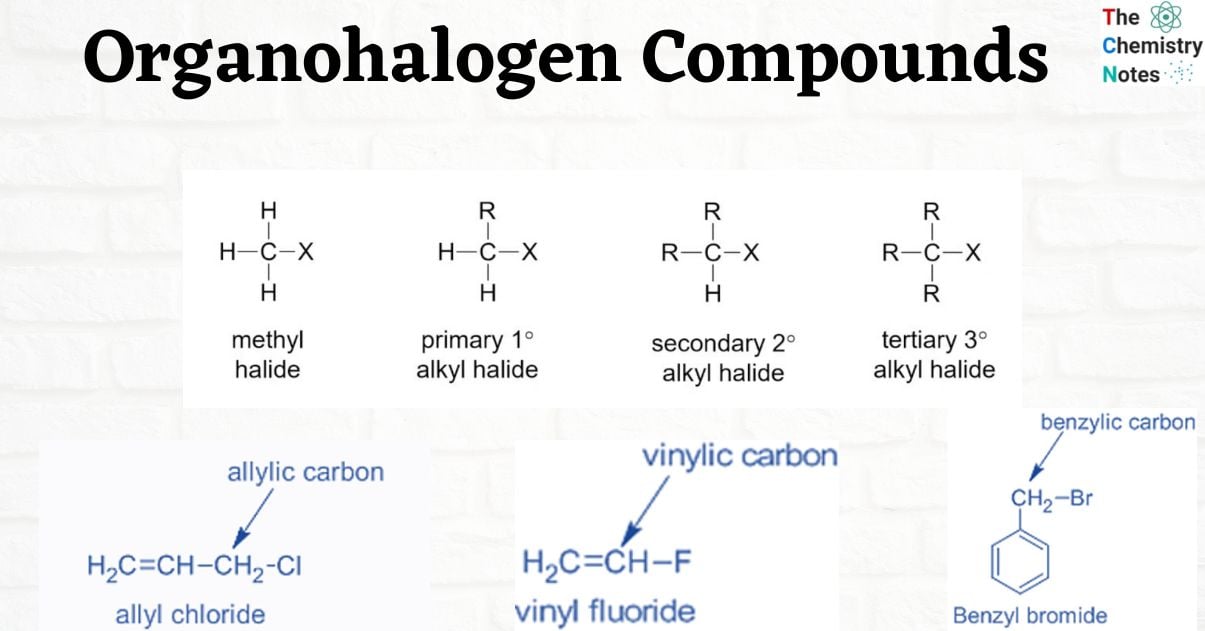
Classification of Organohalogen Compounds
The following are the class of organohalogen compounds
Classification based on sp3 C–X Bond
- Alkyl halides (also known as haloalkanes)
- Allylic halides
- Benzylic halides
Alkyl Halides
R- X. Halogen, X, [X can be F, Cl, Br, or I] is directly bound to sp3 carbon.
Examples: Methyl halides (CH3Cl)- halide is attached to a methyl group.
Common name: Methyl Chloride
IUPAC name: Chloromethane
Alkyl Halides are further classified as primary (1°), secondary (2°), or tertiary (3°) based on the type of carbon to which the halogen is linked.
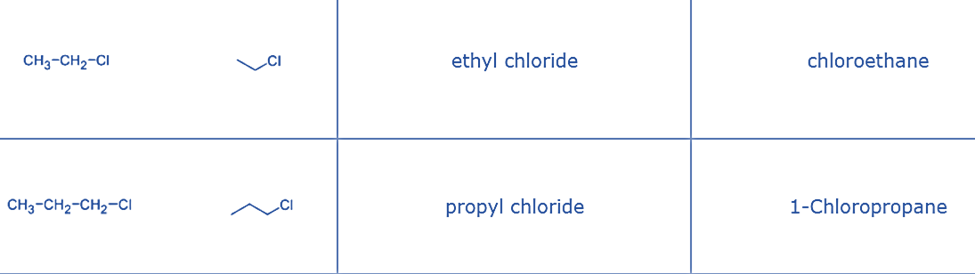
- Primary alkyl halide: ethyl chloride – the chlorine atom is attached to primary carbon.
- Secondary alkyl halide: isopropyl chloride – the chloro group is attached to a secondary carbon.
- Tertiary alkyl halide: tert-butyl chloride – the chlorine atom is bonded to a tertiary carbon.
| Structure | Name | Class |
| CH3CH2Br | bromoethane | primary alkyl halide |
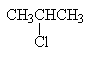 | 2-chloropropane | secondary alkyl halide |
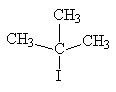 | 2-iodo-2-methylpropane | tertiary alkyl halide |
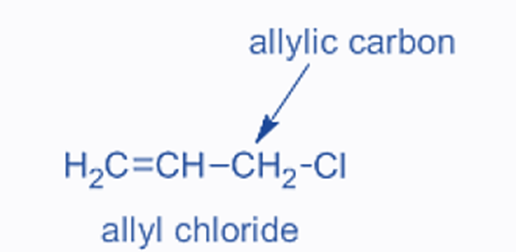
Allylic halides
The halogen in allylic halides is bound to an sp3-hybridized carbon atom that is close to a C=C. This carbon is also referred to as allylic carbon.
Benzylic halides
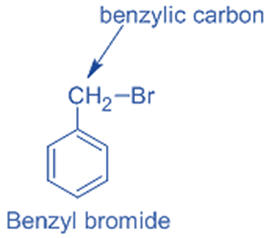
In benzylic halides, the halogen group is joined to a benzylic carbon atom that has undergone sp3 hybridization and is then joined to an aromatic ring.
For example, benzyl bromide.
Classification based on sp2 C–X Bond
- Vinylic halides
- Aryl halides
- Acid halides
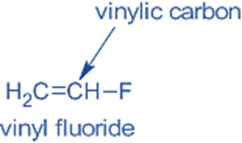
Vinylic halides
The sp2-hybridized carbon of a C=C is directly bonded to the halogen atom.
For example, vinyl fluoride.
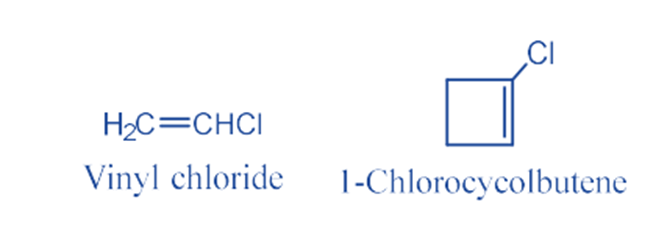
Other Examples:
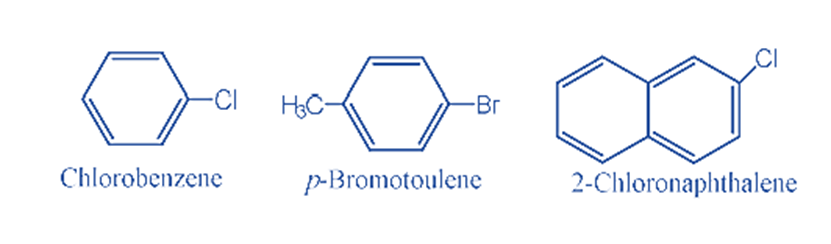
Aryl halide (Haloarene)
Haloarenes, commonly referred to as aryl halides are a class of organic compounds in which the halogen atom is directly attached to the sp2-hybridized carbon atom of an aromatic ring.
For example:
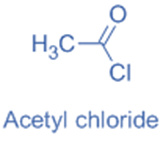
Acid halides
Acid halides are compounds that are derived from carboxylic acids. Halogenated carboxylic acids are produced by replacing the hydroxyl (-OH) group of a carboxylic acid with a halogen atom. The X atom is covalently bonded to a carbonyl carbon that has undergone sp2 hybridization in this instance.
Polyhalogenated Compounds
Polyhalogenated compounds are organic compounds that contain multiple halogen atoms. Organohalogen compounds may comprise one or multiple halogens. These compounds are commonly known as dihalo, trihalo, tetrahalo, and polyhalo compounds.
Dihalo-substituted Compounds
Compounds that have been substituted with two halogen atoms are referred to as dihalo-substituted compounds. For instance, the chemical compound known as methylene chloride is represented by the molecular formula CH2Cl2.
Geminal Dihalides: When two halogen groups are located on a single carbon atom, they are classified as geminal dihalides. For instance, Ethylidene dichloride is a geminal dihalide.
Vicinal Dihalides: On the other hand, when two halogen atoms are present on neighboring carbon atoms, they are known as vicinal dihalides. Ethylene dichloride is an example of a vicinal dihalide.
Trihalo Substituted
Trihalo substituted halogen compounds refers to an organic compound in which three halogen atoms are substituted for other atoms or groups of atoms in a molecule.
For example:
- Chloroform (CHCl3)
- Westrosol (or Trichloro-ethylene) (CHCl = CCl2)
Tetra halo-substituted
The term “tetra halo-substituted” refers to a compound that has four halogen atoms substituted onto its molecular structure.
Example:
- Carbontetrachloride (CCl4)
- Westron (or Acetylene tetrachloride) (CHCl2-CHCl2)
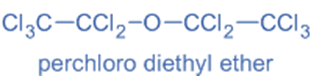
Perhalo compounds
Perhalo compounds are a type of chemical compound in which all hydrogen atoms are replaced by halogen atoms, typically referred to as X atoms.
For example: perchloro diethyl ether.
Nomenclature of Organohalogen Compounds
Organohalogen compounds are named using two types of IUPAC nomenclature, namely substitutive and functional class.
- Substitutive nomenclature involves the addition of a prefix such as fluoro-, chloro-, bromo-, or iodo- to the name of the hydrocarbon framework. This prefix is accompanied by a locant, which is a number that identifies the specific carbon atom to which the halogen is attached. The order of listing substituents, such as halogens, is based on alphabetical order.
- Functional class nomenclature employs two distinct terms to designate alkyl halides. The initial term denotes the IUPAC nomenclature of the alkyl group (as defined in hydrocarbon), while the subsequent term specifies the halogen present, namely fluoride, chloride, bromide, or iodide. The numbering of the alkyl group chain commences at the carbon atom to which the halogen is bonded.
Properties of Organohalogen Compounds
- The trend in electronegativity of halogens is observed to decrease as one moves down the group from fluorine to iodine. Consequently, the polarity of the bonds between these elements and other elements exhibits variation.
- The bonds formed between halogen atoms and carbon exhibit a comparable trend, wherein the bonds that are most polarized are those formed between carbon and fluorine. Conversely, molecules that possess carbon to halogen bonds exhibit distinct dipole moments.
- The presence of fluorine or chlorine in certain cases can result in significant molecular dipole moments, which can have a profound impact on the solubility of a compound in water as well as its melting and boiling points.
References
- https://unacademy.com/content/jee/study-material/chemistry/halogenated-organohalogen compounds/
- https://www.simply.science/images/content/chemistry/organic_chemistry/halogen_derivatives/conceptmap/organohalogen.html
- https://www.sydney.edu.au/science/chemistry/~george/halides.html
- https://www.britannica.com/science/organohalogen compound
- https://faculty.ksu.edu.sa/sites/default/files/1_chem_340_halogen_compounds_0.pdf
