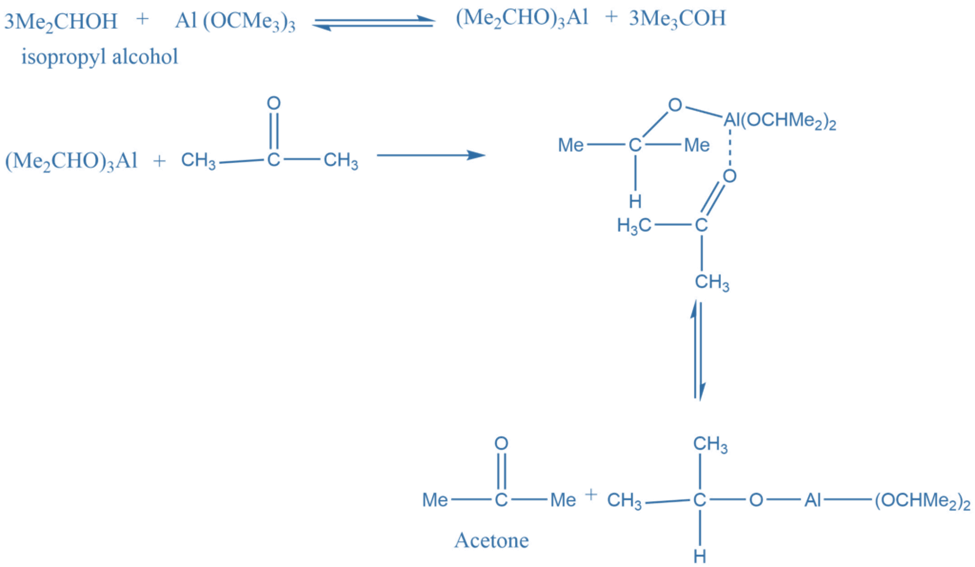
Oppenauer oxidation is a chemoselective oxidation reaction that involves the oxidation of secondary alcohols to ketones in presence of aluminium tertiary butoxide in benzene or toluene solution. The reaction, which is named after Rupert Viktor Oppenauer, is one of the most beneficial in modern chemistry. It is the oxidation of a secondary alcohol to the corresponding ketone catalyzed by aluminium alkoxide. A large excess of the ketone is used to proceed with the reaction in the desired direction. It is the reverse of Meerwein-Ponndrof-Verley reduction. THe overall reaction mechanism is:

Interesting Science Videos
Reaction mechanism Of Oppenauer Oxidation
In the first step, alcohol forms a complex with aluminium isopropoxide. This complex on reaction with a ketone gives a six-membered transition complex. The aluminium-catalyzed hydride shift converts the alcohol’s alpha-carbon to the carbonyl carbon. The desired product is formed by the hydride shift.
Apllications Of Oppenauer Oxidation
- It is used to oxidize alcohol when there is another oxidizable functional group because it is only specific for alcohols (olefinic bond, phenolic group).

2. It is used to convert formates into a carbonyl group.

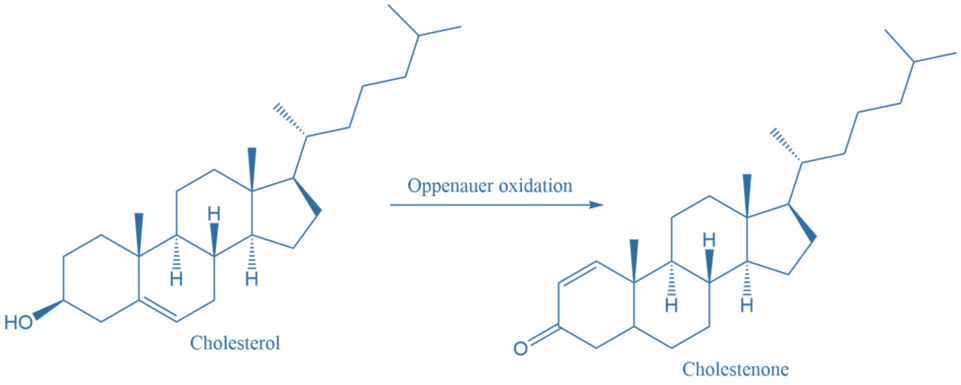
3. Cholesterol is oxidized to cholestenone by using this reaction mechanism.
Limitations Of Oppenauer Oxidation
- This reaction requires high temperature. Aldol condensation products that are formed with hydride acceptors as the end product.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
