Nuclear reactions are the process in which two atomic nuclei or one atomic nucleus and a subatomic particle (such as a proton or neutron or high energy electron) collide to form one or more nuclides different from parent nuclei.
In these reactions, the nuclei, of the reactants interact with each other resulting in the formation of new elements. This process is also called the transmutation of nuclei. Generally, the nuclear reaction involves two reacting particles – a heavy, target nucleus and the light bombarding particle. These two reacting particles react with each other to produce two new particles, a heavier product nucleus, and a lighter ejectile particle.
Lord Rutherford discovered the phenomenon of nuclear transmutation or nuclear reaction in 1919. He bombarded nitrogen gas with 7.7 M eV high-energy α -particles obtained from a polonium source. The ejected lighter particles were identified as hydrogen nuclei or protons (11H or p), and the product nuclei as a rare oxygen isotope.
Protons, deuterons, neutrons, and other light nuclei can be used to produce nuclear reactions. Neutrons are the best projectiles for producing nuclear reactions because they are neutral particles and do not experience Coulomb repulsion. So, even thermal neutrons (neutrons with an energy of 0.0253 eV) can enter the target nucleus and cause a nuclear reaction.
Examples of nuclear reactions:
63Li + 11H → 32He + 42He
105B + 1on → 73Li + 42He
Classification of Nuclear reactions
Nuclear reactions are classified into two types they are:
1. Nuclear reactions, based on overall energy transformation that occurs during nuclear reactions.
2. Nuclear reactions, based on the nature of bombarding particles used to bombard the target nucleus.
Nuclear reactions, based on overall energy transformation
Based on overall energy transformation nuclear reactions are classified into different types. They are:
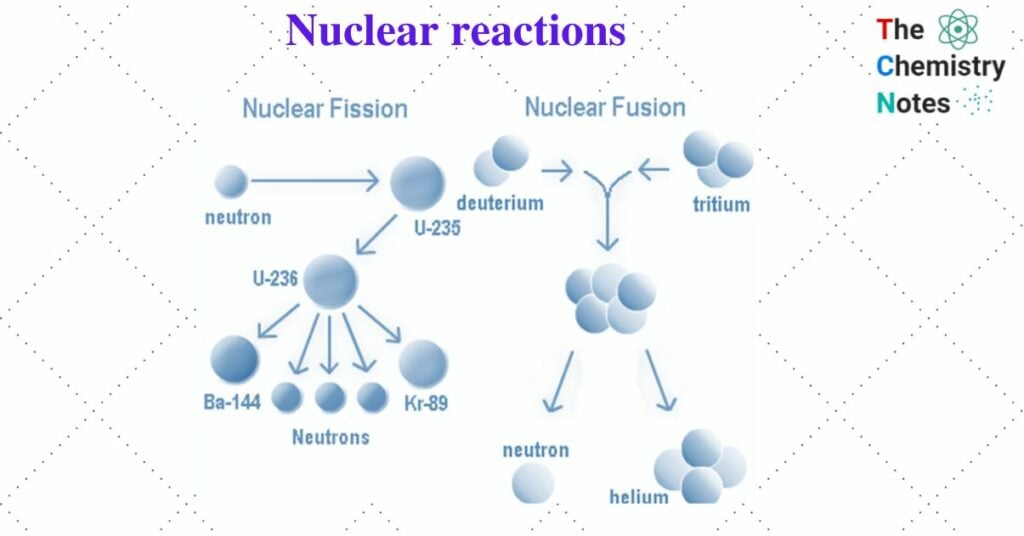
I. Fission reaction
Large nuclei (mass number greater than 230) are unstable and can split into two or more parts. This is known as nuclear fission. In the fission process, when a nucleus interacts with a projectile (bombarding particle), it splits into two or lighter nuclei of roughly similar mass, releasing a large amount of energy and emitting a few neutrons.
If the released neutrons are slowed down by a moderator (such as graphite, heavy water, or beryllium that causes neutrons to travel more slowly), they can be absorbed by other nuclei. If these neutrons are absorbed by other nuclei, a chain reaction occurs. The neutrons i.e., 1on are typically used in the fission of heavier nuclei such as U, Th, and Ra, whereas charged particles such as 11H, 21D, and 42He are generally used in the fission of lighter nuclei.
For example, when 23592U is bombarded with slow-moving neutrons, it breaks up into two fragments 14456Ba and 9036Kr, and the number of neutrons is also liberated which can bring fission of another 23592U and so on.
23592U + 1on → 14456Ba + 9036Kr + 2 10n + 200 Mev
When nuclear fission occurs, a massive amount of energy is released, which is the principle of the atom bomb.
II. Fusion reaction
Two lighter nuclei combine to form a heavier nucleus. This is known as a nuclear fusion reaction. Lighter nuclei have lower binding energy and are thus less stable. As a result, two or more such nuclei have a tendency to combine or fuse, resulting in a nucleus with a higher atomic mass, higher binding energy, and higher stability. In this reaction, a tremendous amount of energy is released which is the principle of the hydrogen atom.
21H + 31H → 42He + 10n + 17.6 Mev
The colliding nuclei must have enough kinetic energy to overcome the initial repulsion between the positively charged cores for this reaction to occur. This energy can be made available by heating the reaction system to several million degrees. As a result, such reactions are known as thermonuclear reactions. The high temperature required to initiate a nuclear fusion reaction also can be attained initially through the fission process (i.e., from atom bomb).
III. Spallation reaction
Spallation reactions occur when a high-energy projectile (typically a proton, neutron, or alpha particle) fragments a heavy nucleus, removing one or more nucleons and producing lighter nuclei. The term spallation is derived from the verb “to spall” i.e., to break up by chipping off small fragments from the bigger one. In 1947, Seaborg and a coworker discovered that bombarding target nuclei with high-energy particles can break target nuclei into smaller nuclei and a large number of light particles (nucleons). Spallation may occur with light as well as heavy nuclei. In heavy nuclei, fission and spallation occur at the same time. These reaction processes are distinguished by the mass distribution between fragments obtained. In fission, masses are very much smaller than the target whereas, in spallation, the masses differ by up to 10 to 20 units from that of the target.
For example, the bombardment of 6329 Cu nuclei by a high-speed α -particle having energy 400 MeV gives 3717Cl nuclei, 14 protons, and 16 neutrons.
6329Cu + 42He → 3717Cl + 14 11H + 16 10n
IV. Projectile capture reactions
In this reaction, the projectile (bombarding particle) is captured or absorbed by the target nucleus to form the product nucleus.
8537Rb + 10n → 8637Rb + γ
5927Co + 10n → 6027Co
γ – radiations may or may not be emitted.
V. Projectile capture particle emission reactions
In this reaction, the projectile (bombarding particle) is captured or absorbed by the target nucleus to form the product nucleus in addition to the emission of a massive particle.
147N + 10n → 146C + 11H
115B + 11H → 116C + 10n
Classification based on the nature of bombarding particle
This classification gives different types of nuclear reactions that can be carried out by using different projectiles like a proton (11H), neutron ( 10n), deuteron ( 21H), α -particles, and γ- ray
I. Nuclear reactions induced by α – particles
α – Particles being doubly charged will have high potential barriers and are hence suitable for low atomic weight target nuclei. With high atomic weight targets, they will be dispersed. So sufficiently energized α – ray can be used as a projectile for the atomic weight target nucleus.
The reactions can be further classified based on the particle which is emitted.
E.g., 147N + 42He → 178O + 11H (α, p reaction)
In this reaction α -particle 42He is used as a projectile and a neutron (10n) is emitted. So, the reaction is (α, p) reaction. Similarly, if the n and γ were emitted then it is called (α, n) and (α, γ) reactions.
E.g.,
126C + 42He → 158O + 10n (α, n reaction)
73Li + 42He → 115B + γ (α, γ reaction)
II. Nuclear reactions induced by protons
Different types of nuclear reactions occur when protons act as projectiles.
2311Na + 11H → 2312Mg + 10n (p, n reaction)
2713Al + 11H → 2814Si + γ (p, γ reaction)
III. Nuclear reactions induced by neutrons
In these reactions, the neutrons are captured by target nuclei resulting in the emission of the particles like γ – rays, neutrons, protons, and α particles.
2713Al + 10n → 2813Al + γ (n, γ reaction)
63Li + 10n → 31H + 42He (n, α reaction)
IV. Nuclear reactions induced by photons or γ – rays
21H + 00γ → 11H + 10n (γ, n reaction)
94Be + 00γ → 84Be + 10n (γ, n reaction)
V. Nuclear reactions induced by deuterons
It is a more effective projectile than either 11H or 42He.
73Li + 21D → 83Li + 11H (d, p reaction)
94Be + 21D → 84Be + 31T (d, T reaction)
Group displacement law
Nuclear reactions are associated with certain kinds of emissions. Each nuclear disintegration is characterized by the nature of the emission given out in that step. The emission of α and β particles are common in nuclear reactions. The element emitting the α or β – particle is known as the parent element, and the new element formed is known as the daughter element.
In 1913, Fajan, Russel, and Soddy stated the effect of an α- and β- particles on the nature of the daughter element in the form of a law which is known as the group displacement law. This law state as follows:
I. In the radioactive transformation, only one α- and β- particle will be emitted at a time.
II. When α- particle is emitted by a radioactive element, the new element formed has a mass number less by 4 units and an atomic number less by 2 units. So, the daughter element formed gets shifted two columns to the left in the periodic table.
21584Po (Group no.16 ) → 21182Pb (Group no.14) + α
III. When β -particle is emitted by a radioactive element, the new element formed has the same mass number as the parent element, but its atomic number increases by one unit. As a result, the daughter elements are shifted one column up to the periodic table. So this law helps to fic the position of radioactive elements in the periodic table.
Some important points
1. α – Decay results in the formation of an isobaric element i.e., parent and daughter nuclides have different atomic numbers but the same mass number.
E.g.,
4019K → 4020Ca + 0-1e
2. Although the – electron is not present in the nucleus, it is emitted from the nucleus because a neutron first breaks down into a proton and an electron.
10n → 11P + 0-1e
Applications of nuclear reactions
- Nuclear energy is an excellent source of process heat for a variety of industrial applications such as synthetic oil production and oil refining.
- Radioisotope power sources have been an important source of energy in space.
- Nuclear energy is extremely beneficial; for nuclear-powered ships.
- Industrial radiography and mineral analysis using sealed radioactive sources.
- Radioactive tracing employs short-lived radioactive materials.
- Radiotherapy can be used to treat medical conditions such as cancer.
References
- https://byjus.com/chemistry/nuclear reaction/#:~:text=Nuclear%20reactions%20are%20processes%20in,to%20as%20the%20parent%20nuclei).
- ht tp://websites.umich.edu/~ners311/CourseLibrary/bookchapter17.pdf
- http://sarsunacollege.ac.in/WebPages/Downloads/ELearning/Science/Physics/UG%204th%20Sem/fission_and_fusion_teacher_notes.pdf
- https://nios.ac.in/media/documents/SrSec312NEW/312_Physics_Eng/312_Physics_Eng_Lesson27.pdf
- https://link.springer.com/referenceworkentry/10.1007/978-3-662-44185-5_1476
- https://data.testprepkart.com/Online_Preparation/JEE/JEE%20Chemistry/7_JEE_Chemistry_Nuclear%20Chemistry/4_JEE_Chemistry_Nuclear%20Chemistry_Group%20displacement%20law.pdf
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_General_Chemistry%3A_Principles_Patterns_and_Applications_(Averill)/24%3A_Nuclear_Chemistry/24.03%3A_Nuclear_Reactions
