Nitrobenzene is the most basic aromatic nitro compound, with the molecular formula C6H5NO2. Nitrobenzene was initially synthesized in 1834 by the German chemist Eilhardt Mitscherlich, who used fuming nitric acid to treat benzene. It is an oily, pale yellow, or colorless liquid with the scent of bitter almonds. It is frequently employed in the synthesis of aniline, benzidine, and other organic compounds.
Interesting Science Videos
Preparation of nitrobenzene
In the laboratory, nitrobenzene is prepared by the nitration of benzene. In this process, Benzene is heated to around 60°C with a mixture of concentrated HNO3 and concentrated H2SO4. A nitro group replaces a hydrogen atom in the benzene nucleus, yielding nitrobenzene. During the reaction process, the interaction between the two acids mixtures produces the nitronium ion (NO2+), which is the active species in aromatic nitration. Sulfuric acid is not consumed during mixed-acid synthesis. It serves as a catalyst and a water absorber.
C6H6 + HNO3 → C6H5NO2 + H2O
Procedure
A round bottom flask is filled with a mixture of 60 ml concentrated nitric acid and 60 ml concentrated sulfuric acid. This is referred to as a nitrating mixture. 60 ml of benzene is gradually added to the mixture, with constant shaking and cooling after each addition. After adding all of the benzene, the mixture is heated in a water bath at 60 o C for an hour, until a pale yellow greasy layer of nitrobenzene with a bitter almond aroma develops. A separating funnel is used to separate the nitrobenzene layer.
Thus obtained nitrobenzene may contain acidic impurities. It is then rinsed several times with dilute Na2CO3 to eliminate acidic impurities and then with water. It is then dried with anhydrous CaCl2 and redistilled at 210-215 oC using an air condenser to obtain pure nitrobenzene.

Fig: Preparation of nitrobenzene
Image source: https://sajhanotes.com/grade-12-chemistry/nitroalkane-and-nitrobenzene/
Physical properties of nitrobenzene
- It is a pale yellow liquid.
- It has a boiling point of 211°C.
- It smells strongly of bitter almonds.
- It is water-insoluble but soluble in organic solvents including alcohol, ether, and benzene.
- It has a density of 1.2037. It is also known as mirbane oil.
- It is very hazardous.
- It is team volatile.
Chemical properties of nitrobenzene
Reaction due to benzene nucleus
Because of the presence of the benzene nucleus, this additive, like other aromatic compounds, shows substitution reactions. The nitro group acts as a ring deactivator. The Nitro group is a Meta director group. As a result, meta-products are formed during substitution processes. This can be explained on the basis of the following resonating structure:
It is obvious from the resonance structures that electron density is quite high at the meta position. As a result, the entering electrophile attacks at the meta position to produce meta substituted product. As a result, -NO2 is a meta-directing group.
it undergoes nitration, halogenation, and sulfonation much more slowly than benzene. Depending on the reaction conditions, it can be reduced to a range of compounds.
Chlorination reaction
It reacts with chlorine in presence in the presence of a halogen carrier like FeCl3 to produce meta chloro nitrobenzene.

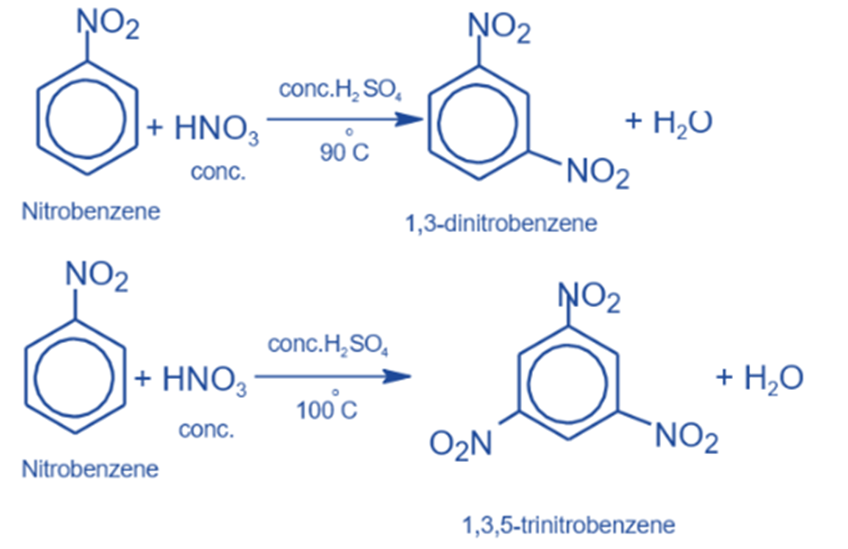
Nitration reaction
When nitrobenzene is heated to around 100°C with a mixture of concentrated nitric acid and concentrated sulfuric acid, it transforms into m-dinitrobenzene. When temperatures rise above 100°C, 1,3,5-Trinitrobenzene (TNB), a highly explosive substance, is produced.
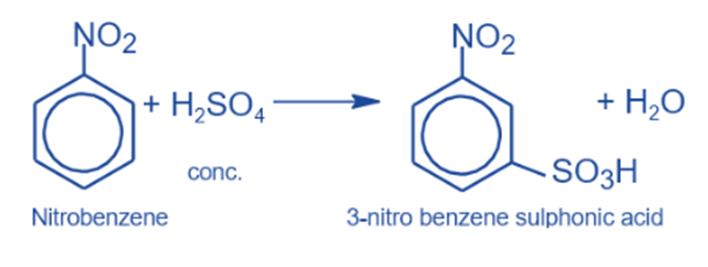
Sulphonation reaction
It produces m-nitrobenzene sulfonic acid when heated to roughly 100°C with mild sulphuric acid.
Recation with a nitro group
It undergoes reduction reactions due to the presence of the nitro group. The nitro group reduces in these processes while the benzene nucleus remains unaffected.
Reduction in acidic medium
It is converted to aniline in an acidic solution using Sn/HCl, Fe/HCl, or Fe/HCl.
C6H5NO2 + 6H → C6H5NH2 + 2H2O
Reduction in neutral medium
It is reduced by Zn and aqueous NH4Cl solution to produce phenol hydroxyl amine. The reaction of an Al-Hg pair or a Zn-Cu pair with water yields the same result.
C6H5NO2 → C6H5NO → C6H5NHOH
Reduction in alkaline medium
It undergoes a bimolecular reduction in an alkaline medium with various reagents to yield various compounds. Hydrazobenzene, for example, is produced by reducing nitrobenzene with Zn and NaOH solutions.
C6H5NO2 + 2H → C6H5NO + H2O
C6H5NO + 2H → C6H5NHOH
Reduction by LiAlH4
It is converted to azobenzene using lithium aluminium hydride.

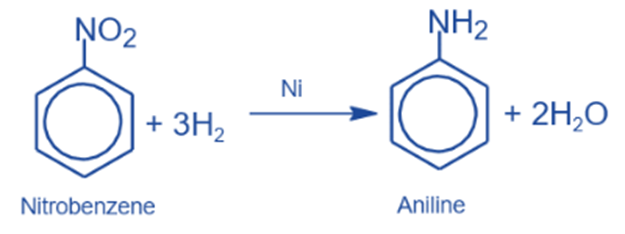
Catalytic reduction
It is converted to aniline in the presence of a catalyst such as Ni, Pt, or Pd.
Electrolysis
It generates p-aminophenol electrolytically in a strong acidic media. Nitrobenzene reduction produces phenol hydroxylamine, which then rearranges in the presence of strong acid to generate p-aminophenol.

Uses of nitrobenzene
- It is utilized in the production of aniline.
- It’s used to make shoe polish.
- It is employed in organic synthesis as an oxidizing agent.
- It is used for scenting inexpensive soaps.
- It is also known as mirbane oil and is used as an inexpensive fragrance in soap and shampoo.
- It is used to make dyes, medicines, and explosives, among other things.
- For the production of shoe polish.
- To make dyes, medicines, explosives, and others.
- In some reactions, it is utilized as an inert solvent with a high boiling point.
Health hazards of nitrobenzene
- It has a number of negative health effects. Direct skin or eye contact can produce a minor irritation.
- Excessive nitrobenzene exposure can result in methemoglobinemia, a blood problem. This impairs the blood’s ability to transport oxygen.
- When exposed to It, the skin may turn bluish, and people may have nausea, vomiting, or breathing difficulties. Additionally, it can cause headaches, grouchiness, dizziness, weakness, or tiredness.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992.
- https://chemistrypage.in/nitrobenzene-structure-and-formula/.
- https://www.britannica.com/science/nitrobenzene.
