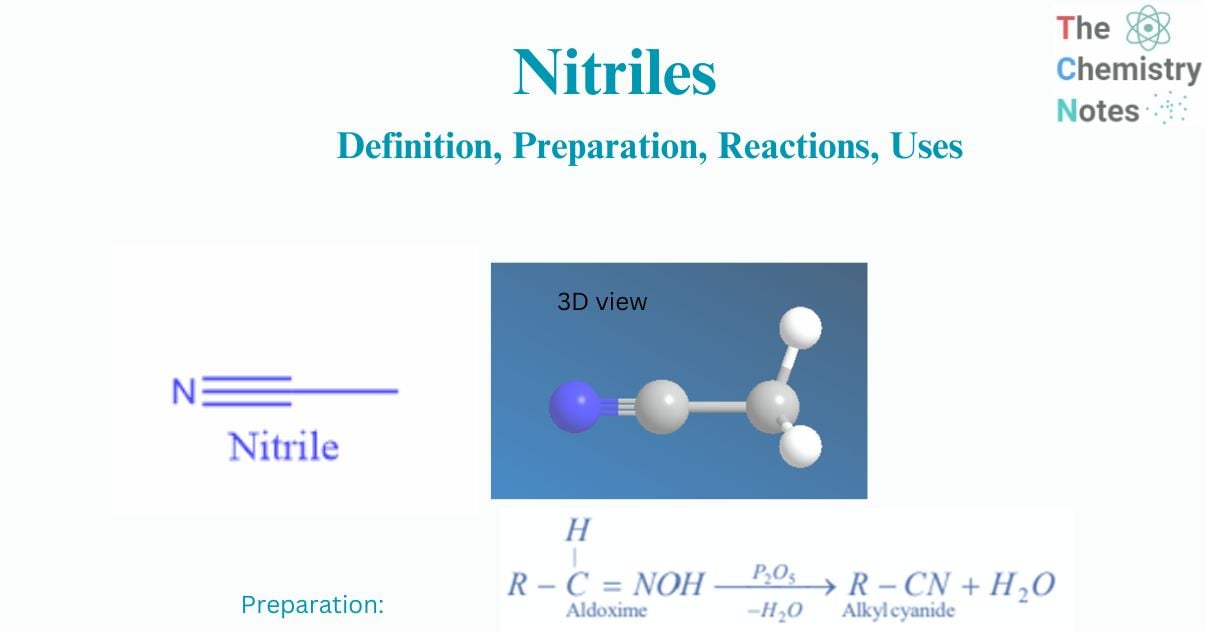
Nitriles are considered a derivative of hydrocyanic acid (HCN). When the hydrogen atom of HCN is replaced by an alkyl or aryl group, the compounds obtained are called cyanides or nitriles.
The organic derivatives of hydrocyanic acid HCN are alkyl cyanides (RCN).
The parent component of nitriles is hydrocyanic acid (HCN). Scheele isolated it in 1782 by acid hydrolysis of a glycoside known as ‘Amygdalin’. Hydrolysis Amygdalin was converted into benzaldehyde, glucose, and hydrocyanic acid, commonly known as Prussic acid.
C20H27O11N + 2H20 → HCN + C6H5CHO + 2C6H12O6
Amygdalin Hydrocyanic acid Benzaldehyde Glucose
HCN is also formed when sodium cyanide is heated with concentrated sulphuric acid-
NaCN + H2SO4 → HCN + NaHSO4
Hydrocyanic acid can exist in two tautomeric forms ie., HCN And HNC so it can form two kinds of alkyl derivatives i.e., alkyl cyanides and alkyl isocyanides.
Cyanides are a suitable synthetic pathway for preparing amides, acids, and amines, so they are employed to make these chemicals. Nitriles can be utilized as intermediates in organic reactions to increase the number of carbon atoms in the chain, as one extra carbon atom is added due to the conversion of alkyl halides to alkyl cyanide. Acetonitrile (CH3CN) is a common industrial solvent that is also used in the production of textiles, nitriles, rubber, and other materials. Cyanides have a pleasant odor, whereas isocyanides have a very unpleasant odor. Cyanide has a greater dipole moment than isocyanides.
Interesting Science Videos
Nomenclature of Nitriles
Common Name System
Cyanides are named in two ways in the common name system.
- The suffix cyanide is added to the name of the alkyl or aryl group in the first technique.
For example CH3CN (methyl cyanide), C2H5CN ( ethyl cyanide)
- In this system, the suffix -onitrile replaces -ic acid in the common name of the carboxylic acid that the alkyl cyanide produces upon hydrolysis. As an example,
CH3CN + 2H2O CH3COOH + NH3
Acetic acid Acetonitrile
IUPAC Name System
- As the parent chain, the longest chain of carbon atoms containing the -CN group is chosen.
- The carbon of the -CNgroup is counted in addition to the carbon of the parent chain.
- As a result, the parent alkane is identified, and the suffix ‘nitrile’ is added to the parent alkane’s name.
- For example, CH3CN ethanenitrile.
• The carbon atom triply connected to the nitrogen atom is designated as 1 in substituted nitriles.
CH3-CH2-CH(CH3)-CN 2- methyl butane nitrile
- If -CN is a substituent in a compound with more than one functional group, it is designated by the prefix cyano.
CH3-CH2-CH(CN)-COOH 2- cyano butanoic acid
- Cyclic nitriles are named by attaching the suffix carbonitrile to the name of the ring to which the -CN group is connected.
Methods of Preparation of Nitriles
From alkyl halide
Alkyl halide when warmed with ethanolic solution of potassium cyanide or sodium cyanide, produce alkyl nitriles. An SN2 reaction between an alkyl bromide and sodium cyanide produces nitriles.
R−X + KCN → R−CN + KX
CH3−Cl + KCN → CH3−CN + KCl
Instead of nitrile, alcohol can be generated when an aqueous solution of potassium cyanide (KCN (aq)) is heated under reflux with the haloalkanes.
Tertiary halides quickly break down into alkenes and hydrogen halides when heated, making it impossible to manufacture tertiary cyanides using this method.
From amides
Amides on heating with strong dehydrating agents like P2O5, SOCl2, or POCl3 produce nitriles. Nitriles are of great importance in organic synthesis because, unlike amides, can undergo a number of essential reactions.
RCONH2 + SOCl2 → RCN + H2O
From carboxylic acid
Alkyl cyanides are produced industrially by passing a mixture of carboxylic acid and ammonia over alumina at 500°C.
RCOOH + NH3 → RCOONH4 → RCONH2 → RCN
From Grignard reagent
In the presence of dry ether, Grignard reagent (RMgX) reacts with cyanogen chloride (ClCN) to give alkyl nitrile.
RMgX + ClCN → RCN + MgXCl
This method is employed for the preparation of tertiary alkyl nitrile.
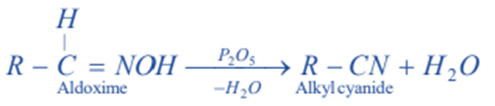
From aldoximes
Aldoximes when dehydrated with P2O5 or acetic anhydride give corresponding nitriles.
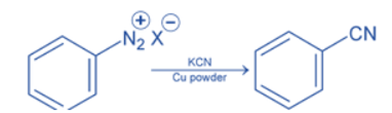
From diazonium salt
Diazonium salt reacts with KCN or NaCN in the presence of cuprous cyanide (CuCN) or copper powder as a catalyst. This method is only applied for the preparation of aryl cyanide.
Physical Properties of Nitriles
- Alkyl cyanides are stable, neutral chemicals with a pleasant odor. Lower members up to C14 are liquids, whereas higher members are crystalline solids. The toxicity of alkyl cyanides is lower than that of hydrogen cyanides.
- Lower alkyl cyanides are fairly soluble in water, whereas ethanenitrile is completely soluble. Nitriles can form hydrogen bonds with water molecules even if they cannot hydrogen bond with other nitriles, which accounts for their solubility. A hydrogen bond is established when one of the slightly positive hydrogen atoms in a water molecule is attracted to the lone pair on the nitrogen atom in a nitrile. The solubility decreases with an increase in chain length, This is due to an increase in the hydrophobic part of nitriles.
- Cyanides have a greater boiling point than halides. For example, CH3CN is 335 K, while CH3Cl is 249.5 K.
The -CN group is extremely polar. As a result, the dipole moments of alkyl cyanides are very large. As a result of the strong intermolecular dipole-dipole interaction, the alkyl cyanides have a considerably higher boiling point.
- Nitriles exhibit strong dipole-dipole motions as well as Van der Waals dispersion forces between molecules.
- They also have a high polarity and electronegativity.
Reactions of Nitriles
Hydrolysis
Alkyl cyanides can be hydrolyzed by both dilute acid and dilute alkali. to give carboxylic acid. The reaction proceeds through the production of an amide intermediate. Hydrolysis can be stopped at the amide step under mild conditions.
RCN → RCONH2 → RCOOH
Reduction
The final product of the reduction reaction depends on the reaction conditions.
- Complete reduction
When alkyl cyanides are reduced with hydrogen in the presence of Pt or Ni, LiAlH4 (Lithium aluminum hydride), or sodium and alcohol, they produce primary amines.
RCN + 4 H → RCH2NH2
The reduction of cyanides to primary amines by using LIALH4 or Na/C2H5OH is known as the Mendius reaction.
- Partial reduction
Imine hydrochloride is precipitated when hydrogen chloride is passed through an ethereal solution of cyanide in the presence of stannous chloride at room temperature. When imine hydrochloride is heated with water, it hydrolyzes to produce the equivalent aldehyde. This is known as Stephen’s reduction.
SnCl2 + 2 HCl → SnCl4 + 2H
RCN + 2 H + HCl → RCH=NH.HCl
RCH=NH.HCl → RCHO + NH4Cl
Reaction with Grignard reaction
Grignard reagent reacts with alkyl cyanides to generate an intermediate imino salt. Which, when hydrolyzed, produces ketone.
R-CN + RMgX → R2C= NMgX → R2C= O + Mg (NH2)X
Alcoholysis
The reaction of alkyl nitrile with alcohol in the presence of hydrochloric acid gives ester. This reaction proceeds through the imido ester intermediate.
RCN + ROH + HCl → RCOOR + NH4Cl
Reaction with H2S
Alkyl nitriles react with H2S to give thioacetamide.
RCN + H2S → RCSNH2
Uses of Nitriles
- Nitriles are employed in the production of nitrile gloves, seals, and hoses due to their chemical resistance.
- They are used in oil-resistant substances as well as low-temperature applications.
- They can be used for organic synthesis reactions.
- Pericyazine, a nitrile molecule, is utilized as an antipsychotic in the treatment of opiate dependence.
References
- March J. (1977). Advanced organic chemistry : reactions mechanisms and structure (2d ed.). McGraw-Hill.
- Morrison R. T. & Boyd R. N. (1983). Organic chemistry (4th ed.). Allyn and Bacon.
- http://www.mugberiagangadharmahavidyalaya.org/images/ques_answer/1589209049CamScanner%2004-21-2020%2020.34.52.pdf.
- https://www.toppr.com/ask/en-np/content/concept/cyanides-and-isocyanides-preparation-physical-properties-and-uses-203129/.
- https://www.chemguide.co.uk/organicprops/nitriles/background.html
