Neptunium, which belongs to the actinide class of the Periodic Table, has an atomic number of 93. It lies between plutonium (94) and uranium (92). It was the first transuranium element to be created intentionally. The radioactive metal neptunium is silvery, ductile, and oxidizes in air. Neptunium is extremely risky to handle because it is both toxic and pyrophoric. After extended exposure, it is also preserved in human bones. Edwin McMillan and Philip Abelson at the University of California successfully created neptunium for the first time in 1940 by subjecting uranium-238 to a neutron bombardment.
Interesting Science Videos
History of Neptunium
- Dmitri Mendeleev created the periodic table and included blank spaces for undiscovered elements, such as one after uranium, the final known element with an atomic number of 92.
- A gap following uranium was still visible in Kasimir Fajans’ 1913 version of the periodic table, indicating the possible existence of a new element.
- Enrico Fermi experimented with induced radioactivity by bombarding known materials with neutrons in 1933. He discovered that heavier atoms release gamma rays, which results in beta decay and an increase in atomic number.
- The neutron bombardment of uranium by Fermi created an isotope with the atomic number 93. However, his claim was challenged because he was unable to isolate the newly produced element.
- Edwin McMillan used a cyclotron to bombard uranium in 1939, creating a new element with a 2.3-day half-life. This element has a distinct chemical, comparable to uranium.
- McMillan successfully isolated the source of element 93 with the help of Philip H. Abelson and reported their findings in 1940.
- Following in the footsteps of Uranium (named after the planet Uranus), element 93 was given the name neptunium. The name Neptune reflects its origins.
Occurrence of Neptunium
- Np is only found on Earth as a byproduct of other elements’ breakdown. It exists due to the disintegration of heavier elements over time.
- Any primordial neptunium that may have existed in the past has already decomposed due to its relatively short half-life.
- Np isotopes can be found naturally in uranium ores. These isotopes form as byproducts of uranium transmutation processes.
- The vast bulk of neptunium on Earth today is created through manmade nuclear processes.
- These reactions, which frequently occur in nuclear reactors, lead to the formation of neptunium isotopes.
Elemental Properties of Neptunium
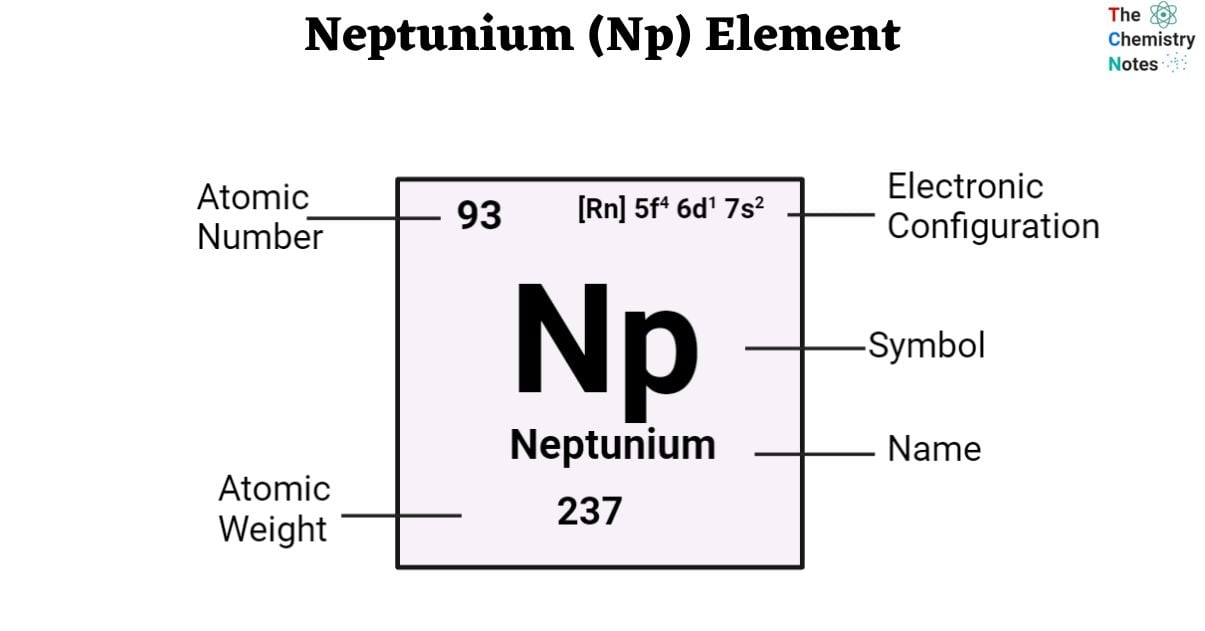
| Electronic Configuration | [Rn] 5f4 6d1 7s2 |
| Atomic Number | 93 |
| Atomic Weight | 237 g.mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | Actinides, 7, f-block |
| Density | 20.25 g/cm3 at 20 °C |
| Ionic radius | 116 pm |
| Van der Waals radius | – |
| Electron shells | 2, 8, 18, 32, 22, 9, 2 |
| Electrons | 93 |
| Protons | 93 |
| Neutrons | 144 (Varies with isotopes) |
Isotopic information of Neptunium
- Np is reported to have 25 radioactive isotopes with atomic weights ranging from 225 to 244, including 5 metastable isotopes.
- The most stable isotopes are Np-237 (half-life: 2.14 million years), Np-236 (half-life: 154,000 years), and Np-235 (half-life: 396 days).
- The half-lives of the majority of neptunium isotopes are less than 4.5 days, with many lasting less than 50 minutes.
- Lighter isotopes, such as Np-237, generally undergo electron capture with substantial alpha emission, yielding largely uranium isotopes.
- Isotopes heavier than Np-237 decay largely through beta decay, resulting in plutonium.
- All neptunium isotopes are radioactive, with neptunium-237 having the longest half-life (2,144,000 years).
- With a half-life of more than 2 microseconds, neptunium-225 is one of the most unstable isotopes.
- Neptunium-237 can be extracted from used reactor fuel for scientific research into the element’s physical and chemical properties.
Physical Properties of Neptunium
- Neptunium is the first transuranic element and a member of the actinide series. Transuranic elements are intrinsically unstable and decay radioactively.
- Np is represented in the periodic table by the symbol Np and has the atomic number 93.
- Neptunium is a radioactive metal that appears hard and silvery metallic. When it is exposed to air, it tarnishes.
- Neptunium-237, like plutonium and uranium, is an alpha emitter, releasing particles composed of two protons and two neutrons after disintegration, similar to a helium nucleus.
- Np is created by bombarding a uranium nucleus with neutrons.
- Plutonium-238 is created by employing neptunium-237 as a target in research reactors, and it is an important source of energy for space missions.
- Neptunium-237 can be found in nuclear waste, usually in combination with other fission products.
- Np exists in three different allotropic forms, each with a different atomic configuration. The element has an orthorhombic crystal structure at room temperature. Above 280oC, this changes to a tetragonal shape, and above 577oC, it changes to a cubic structure.
- Npvalence shell contains 7 electrons, which influences its chemical activity.
| Color/physical appearance | Silvery-white, metallic luster, but often tarnishes to a dull gray color. |
| Melting point/freezing point | 917 K (644°C, 1191°F) |
| Boiling point | 4175 K (3902°C, 7056°F) |
| Density | 20.25 g/cm³ |
| Flammability | Little fragments can easily spontaneously ignite in room temperature air. |
| State of matter at room temperature | solid at room temperature |
Chemical Properties of Neptunium
- Np is a highly reactive element. In powdered form, it is pyrophoric at room temperature.
- Np is the heaviest actinide capable of losing valence electrons in stable compounds.
- The most stable solid state is +4, but the +5 valence state is preferred in solution form.
- When it reacts with water, it produces hydroxides.
Chemical Reaction of Neptunium
- The synthesis of Neptunium (4) ions arises from the reduction of Neptunium Oxide.
2NpO2+ + 4H+ ⇌ Np4+ + NpO22+ + 2H2O
- When neptunium oxide is combined with a reducing agent, it produces halides.
NpO2 + ½ H2 + 3HF → NpF3 + 2H2O
Uses of Neptunium
Plutonium Production Precursor:
- Neptunium is an important precursor in the manufacturing of plutonium, which is utilized in a variety of applications, including thermal generators for spacecraft.
Fast Neutron Reactor Fuel Source:
- Neptunium, when used as a fuel source in fast neutron reactors, helps to efficient energy generation via nuclear fission.
Role in nuclear weapons:
- Neptunium is important in nuclear weapons because it is fissionable and can theoretically be used to make nuclear explosions.
High Energy Neutron Detection:
- Neptunium is used in systems that detect high-energy neutrons, demonstrating its use in nuclear detection technology.
Military Purposes:
- As a precursor in the manufacturing of plutonium, neptunium has military applications, particularly in the creation of materials for thermal generators and associated technologies.
Exploration and research:
- Despite its lack of large commercial applications, neptunium remains critical for research and exploratory efforts in nuclear science and technology.
Fissile Characteristics:
- With a critical mass of 73 kg, neptunium has fissile properties, making it theoretically useable in nuclear bombs; nevertheless, no country has yet used neptunium for this purpose.
Electricity Production in Spacecraft:
- The plutonium derived from neptunium is used as a fuel in thermal generators, providing a consistent source of power for spacecraft on long-duration missions.
Health Effects of Neptunium
- Neptunium exposure is linked to a number of health hazards, including an increased risk of bone cancer.
- The gastrointestinal tract has been identified as the principal organ absorbing the majority of the dosage from neptunium exposure.
- The majority of residual neptunium in the body deposits in the bones, with minor accumulation in the liver.
- Laboratory animal studies show “relatively high concentrations” of neptunium in adrenal glands, indicating its dispersion in different organs.
- In human populations, no specific health effects associated with neptunium exposure have been documented.
- When swallowed or inhaled, neptunium is poisonous, posing risks to human health.
- Some neptunium isotopes, such as Np-236 and Np-237, have a low chance of producing considerable external radiation, underscoring the possible dangers of neptunium exposure.
Environmental Effects of Neptunium
Negative environmental impacts of neptunium have not yet been documented. Neptunium is recognized as a particularly long-lasting waste when it occurs as a byproduct of the decay of americium isotopes in smoke detectors, with a half-life of at least 2 million years.
Video on Neptunium
References
- https://testbook.com/chemistry/neptunium
- W. M. Haynes, ed., CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 95th Edition, Internet Version 2015, accessed December 2014.
- Tables of Physical & Chemical Constants, Kaye & Laby Online, 16th edition, 1995. Version 1.0 (2005), accessed December 2014.
- National Center for Biotechnology Information (2023). PubChem Element Summary for AtomicNumber 93, Neptunium. Retrieved December 28, 2023 from https://pubchem.ncbi.nlm.nih.gov/element/Neptunium.
- https://byjus.com/chemistry/neptunium/
- https://www.lenntech.com/periodic/elements/np.htm
- https://www.industrialheating.com/blogs/14-industrial-heating-experts-speak-blog/post/95401-facts-about-the-elements-neptunium
- Emsley, John (2011). Nature’s Building Blocks: An A-Z Guide to the Elements. Oxford University Press. ISBN 978-0-19-960563-7.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.

